Abstract
Myoid hamartomas of the breast parenchyma are extremely rare, benign breast neoplasms. Only 15 cases of the parenchymal myoid hamartoma of the breast have currently been described in the medical literature written in English. A 46-year-old woman presented with a huge right breast myoid hamartoma and synchronous contralateral left periareolar cancer. We discuss the clinical features, the radiologic findings, the pathologic findings and the management of this type of lesion. Surgeons should be aware that myoid hamartoma is a rare benign tumor, but it can be the cause of a palpable mass in the breast.
Breast parenchymal hamartomas comprise approximately 0.7% of all benign tumors of the female breast.(1) Arrigoni et al.(2) in 1971, first described mammary hamartomas as lesions composed of a haphazard overgrowth of mature tissues. Myoid hamartoma is a rare subtype of hamartomas that is characterized by the presence of smooth muscle cells. Only 15 cases of myoid hamartoma have been reported in the English medical literature.(3) and 2 cases have been described in the Korean literature.(4,5)
To the best of our knowledge, breast parenchymal myoid hamartoma with synchronous contralateral breast cancer has not been described. Our case manifested as a huge right breast myoid hamartoma with contralateral left invasive ductal carcinoma, which is unique.
Herein, we report the first case of myoid hamartoma of the breast parenchyma with synchronous contralateral breast cancer, and we review the relevant literatures.
A 46-yr-old woman visited our hospital due to masses in both breasts. She complained of a non-tender huge mass that had been slowly growing in her right breast for more than 3 yr, and she recently noticed a small left breast mass. Physical examination revealed a huge, firm, well circumscribed, painless mass that occupied the whole right breast and a hard, fixed, 2×2 cm mass in the periareolar area of the left breast. There were no skin changes of the breast or nipple retraction and no axillary lymphadenopathy was noted. Skin lesions, such as facial papules, trichilemmomas, oral papules, and palmoplantar keratosis were not present externally.
The patient had no other significant medical history and no family history of breast cancer. She had never undergone a breast examination, such as mammography or ultrasonography. An ultrasound examination showed a huge well-defined, heterogeneous echoic mass in the right breast and a 2×1.4 cm ill-defined, lobular hypoechoic mass in the periareolar area of the left breast. We performed ultrasound-guided core needle biopsy for both breast masses. Results revealed a stromal proliferative lesion of the right breast and an invasive ductal carcinoma of the left breast. A breast MRI showed a 9.2×7.2×7.1 cm mixed signal intensity lesion with variable enhancement in the right breast and a 2.1×1.9×1.5 cm oval heterogeneously early enhancing mass in the periareolar area of the left breast (Figure 1). The patient underwent subcutaneous mastectomy of the right breast and breast conserving surgery with axillary lymph node dissection of the left breast.
The right breast tumor grossly presented as a single, well-demarcated, firm, incompletely encapsulated mass with a smooth external surface. Microscopically, the tumor was exclusively composed of stromal elements. In the peripheral portion of the tumor, the stromal component intermingled with adipose and glandular tissue (Figure 2A). The stromal elements consisted primarily of spindle-shaped cells with eosinophilic cytoplasm and elongated cigar-shaped nuclei, and the cells were aggregated in small fascicles (Figure 2B). Nuclear pleomorphism and mitoses were not present. Immunohistochemically, the stromal spindle cells were strongly positive for smooth muscle actin but they did not express CD34, the S-100 protein, or cytokeratin (Figure 2C). Ductal epithelial cells and stromal spindle cells showed nuclear positivity for the estrogen receptor, progesterone receptor, and phosphatase and tensin homolog deleted on chromosome ten (PTEN) (Figure 2D).
These microscopic and immunohistochemical findings indicated that the spindle cells in the stroma were smooth muscle cells. The ductal epithelial cells and stromal spindle cells showed nuclear positivity for the estrogen and progesterone receptors, and PTEN. We did not perform PTEN mutation study. Invasive ductal carcinoma was identified in the contralateral breast.
These histopathologic and immunohistochemical findings indicated that the case was a myoid hamartoma of the right breast parenchyma with a synchronous invasive ductal carcinoma of the left breast.
After operation, the patient was discharged without significant complications. Two years after surgery, the patient is doing well without recurrence or metastasis.
Myoid hamartomas of the breast are a rare subtype of mammary hamartomas in which a significant smooth muscle component is identified within the stroma of the lesion. These hamartomas have been reported to constitute approximately 0.7% of all benign breast neoplasms and their main characteristic is the variety of tissues they contain.(1) Myoid hamartomas may contain both adipose and fibrous tissues in different proportions.
There are several theories about the origin of myoid differentiation. Some authors have suggested a metaplastic process from the myoepithelium as the origin of the tumor, while others have proposed that the muscle cells originate from stromal myofibroblasts or from the wall of indigenous vessels. Another theory involves differentiation of a stromal cell that can develop into adipose tissue, smooth muscle, or even cartilage, and this may explain the variety of constituents seen in breast stromal tumors, which are characterized by an impressive heterogeneity.(6)
Clinically, myoid hamartomas are usually soft, mobile, well defined masses and they differ only slightly in texture from the surrounding breast parenchyma. Because of this clinical feature, they can be misdiagnosed as fibroadenomas. The typical mammographic findings consist of a well circumscribed lesion containing both fat and soft tissue, surrounded by a thin radiopaque capsule.(7) Although mammography and a core biopsy can be diagnostic in most cases, when the diagnosis of a rare tumor like mammary hamartoma is suspected, excisional biopsy and an immunohistochemical study should be performed to make the definite diagnosis.(8)
Microscopically, these lesions are composed of a mixture of smooth muscle, fibrous stroma, and adipose tissue in varying proportions. Immunohistochemical results always contribute to a differential diagnosis. Immunohistochemical staining shows that most myoid hamartomas are positive for smooth muscle actin, desmin, caldesmon, and vimentin, but they may be negative for cytokeratin and the S-100 protein. In the present case, all histopathologic and immunohistochemical findings indicated that the mass was a myoid hamartoma. The histopathologic differential diagnosis for myoid hamartoma of the breast includes primary leiomyoma and leiomyosarcoma with a striking fascicular arrangement of smooth muscle cells and a lack of epithelioid features.(9) Neoplastic changes in mammary hamartomas are exceedingly rare. Mathers and Shrimankar(10) in 2004, reported a case with lobular neoplasia within a myoid hamartoma of the breast. Cowden syndrome, also known as multiple hamartoma syndrome, is frequently associated with a variety of malignancies, such as breast, thyroid and endometrial cancers.(11) Germline mutations in PTEN occur in 85% of patients with Cowden syndrome.(12,13) In our case, there was no clinical feature of Cowden syndrome and the ductal epithelial cells and stromal spindle cells showed nuclear positivity for PTEN immunohistochemistry.
To the best of our knowledge, this is the first case of myoid hamartoma of the breast parenchyma with synchronous contralateral breast cancer. The treatment of choice for breast hamartoma is surgical excision with tumor clear margins. If appropriate surgery is performed, the prognostic outcome is good.
In summary, myoid hamartoma of the breast parenchyma is extremely rare and frequently goes unrecognized by clinicians and pathologists because this lesion looks like other benign lesions. Awareness of myoid hamartoma of the breast parenchyma will be helpful to differentiate this benign tumor from other benign and physiologic changes in the breast.
Figures and Tables
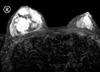 | Figure 1Breast MRI shows a 9.2×7.2×7.1 cm mixed signal intensity lesion with variable enhancement in the right breast (R) and a 2.1×1.9×1.5 cm oval heterogeneously early enhancing mass in the periareolar area of the left breast. |
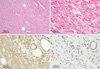 | Figure 2The right breast mass is composed of intersecting bundles of spindle-shaped smooth muscle cells, and involves peripheral ducts and fat tissue (A, H&E stain, ×100). The spindle cells have eosinophilic cytoplasm and elongated cigar-shaped nuclei aggregated in small fascicles (B, H&E stain, ×400). Many spindle cells are strongly positive for actin (C, Immunohistochemical stain, ×100). Ductal epithelial cells and stromal spindle cells show nuclear positivity for PTEN (D, Immunohistochemical stain, ×100). |
References
1. Blomqvist L, Malm M, Fernstad R. Hamartoma of the breast: surgical treatment and reconstruction. Case report. Scand J Plast Reconstr Surg Hand Surg. 1997. 31:365–369.

2. Arrigoni MG, Dockerty MB, Judd ES. The identification and treatment of mammary hamartoma. Surg Gynecol Obstet. 1971. 133:577–582.
3. Stafyla V, Kotsifopoulos N, Grigoriadis K, Bakoyiannis CN, Peros G, Sakorafas GH. Myoid hamartoma of the breast: a case report and review of the literature. Breast J. 2007. 13:85–87.

4. Min DW, Sung SH, Choi IJ. Muscular hamartoma of the breast: a case report. Korean J Pathol. 1994. 28:86–89.
5. Kang BS, Park JM. Muscular hamartoma of the breast: a case report. J Korean Radiol Soc. 2002. 47:99–101.

6. Magro G, Bisceglia M, Michal M, Eusebi V. Spindle-cell lipoma like tumor, solitary fibrous tumor and myofibroblastoma of the breast: a clinico-pathological analysis of 13 cases in favor of a unifying histogenetic concept. Virchows Arch. 2002. 440:249–260.

7. Hessler C, Schnyder P, Ozzello L. Hamartoma of the breast: diagnostic observation of 16 cases. Radiology. 1978. 126:95–98.

8. Rosser RJ. Epithelioid cells in myoid hamartoma of the breast. Arch Pathol Lab Med. 1997. 121:354–355.
9. Garfein CF, Aulicino MR, Leytin A, Drossman S, Hermann G, Bleiweiss IJ. Epithelioid cells in myoid hamartoma of the breast: a potential diagnostic pitfall for core biopsies. Arch Pathol Lab Med. 1996. 120:676–680.
10. Mathers ME, Shrimankar J. Lobular neoplasia within a myoid hamartoma of the breast. Breast J. 2004. 10:58–59.

11. Lloyd KM 2nd, Dennis M. Cowden's disease: a possible new symptom complex with multiple system involvement. Ann Intern Med. 1963. 58:136–142.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download