INTRODUCTION
Neuroendocrine (NE) carcinoma is derived from the NE cells that are scattered throughout all the organ systems, and these NE cells are particularly prevalent in the bronchopulmonary and gastrointestinal tract.(1) Primary NE carcinoma of the breast is extremely rare and it accounts for less than 0.1% of all breast carcinomas, less than 1% of all NE neoplasms in the body and only 0.1% of all breast cancers.(1) However, NE differentiation has also been described in a subset of patients with typical breast carcinoma and the clinical and histopathological features of these patients overlap with those of primary NE carcinoma.(2) Therefore, discriminating pure NE carcinoma of the breast from an ordinary breast carcinoma that shows NE differentiation in focal areas remains difficult.(3) Radiologicaly, it's hard to differentiate pure NE carcinoma from other breast cancers. The prognosis of pure NE carcinoma is better than that for most breast cancers except small cell neuroendocrine tumors. In this report, we present a new cas e of primary NE carcinoma in the breast, and we review the relevant literature with paying particular attention to the general morphological features and the treatment approaches.
CASE REPORT
A 72-yr-old-woman presented at our institution with a painless lump in her left breast that she first noticed a few weeks ago. She described no nipple discharge. On the physical examination, an elastic, firm, indistinct mass with a diameter of 2 cm was palpated in the upper outer quadrant of the breast 3 cm away from the areola. There was no retraction or dimpling, however, axillary lymphadenopathy with any fixation was present.
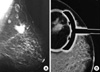
The mammograhy revealed an irregular, lobulated, high density mass (3×2.5 cm) with microcalcifications in the upper outer quadrant of the left breast and this was highly suspicious for malignancy (BI-RADS 5) (Figure 1). The second lesion (1.5×1.5 cm) with a calcified appearance was also noted on the mammogram, and this second leson was adjacent to the first and located in the same area (Figure 1). Ultrasonography (US) also showed two distinct irregular lumps that had heterogeneous echoes with increased vascularity and microlobuted margins, the same as was seen on the mammography. Lymphadenopathy with a minimally thickened cortex was visualised in the axilla and the largest lymph node was measured 23×10 mm. The laboratory data including the serum tumor markers such as CEA, CA 15-3, etc, and the other blood tests were within normal limits. A core needle biopsy of the palpable mass was performed and she was diagnosed with invasive primary neuroendocrine carcinoma. Thus, we performed a modified radical mastectomy with complete axillary lymph node dissection.
A definitive histopathological examination revealed that the tumor was composed of solid nests and sheets consisting of infiltrating small polygonal or oval cells with hyperchromatic nuclei, scant cytoplasm and rare mitoses. The tumor cells were dispersed in solid sheet-like trabecular patterns separated by fibrous stroma (Figure 2A). Papillotubular structures with fibrovascular cores were observed in the nests. The neoplastic cells formed pseudorosettes and palisades at the periphery (Figure 2B). The tumor cells have mildly atypical round to oval nucleus, inconspicuous nucleoli and eosinophilic cytoplasm. Immunohistochemical staining was positive in approximately 60% of the tumor cells for synaptophysin and chromogranin A, (Figure 3A, B). The Ki-67 proliferation index showed very low activity (<10%) in the tumor cells (Figure 3C). Estrogen and progesterone receptors (ER/PR) were nuclear (Figure 4) and E-cadherin was membranous positive. However, the c-erb-B2 expression was negative. It was found that it was a solid neuroendocrine carcinoma. A further work-up that including computed tomography (CT) scan of the thorax and abdomen, an octreotide scan and bone scintigraphy revealed no other abnormalities that can be metastatic disease, a nonmammary primary tumor site or any other malign disease. Based on these findings, the patient was considered to be suffering with primary NE carcinoma of the breast. Histological examination also showed no axillary node metastases to a total of 27 lymph nodes that were dissected during surgery. The patient was scheduled for adjuvant hormonotherapy alone after surgery. Hormonotherapy with tamoxifen was started. The patient is alive and disease free 12 months after surgery.
DISCUSSION
NE carcinomas are relatively slow growing tumors that are derived from NE cells.(1) Primary NE carcinomas of the breast are a group of tumors with specific morphological features that are similar to those of NE tumors that developed in some organ systems other than the breast. The 2003 World Health Organisation (WHO) Classification of Tumors recently recognized primary NE carcinoma of the breast as a distinct entity that has expresses NE markers in more than 50% of the total cell population. From this point of view, the NE differentiation that's observed as focal or scattered areas in human breast carcinomas with additional argyrophilic or carcinoid patterns is not included in this description.(2-4) However, Tavassoli proposed that mammary carcinoma displaying any growth pattern of carcinoid tumor and the tumor shows strongly chromogranin positivity on immunostaining should also be considered as breast carcinoma with NE differentiation.(5) Scopsi et al.(6) reported that argyrophilic breast carcinomas had morphological characteristcs that were similiar to primary NE carcinoma, along with a consistent chromogranin expression.
NE carcinomas of the breast occur mainly in elderly woman who are in the 6th and 7th decade of life, and these patients have no specific clinical features.(2,4,7) Our patient was a 72-yr-old woman whose major compliant was a lump in her left breast. The macroscopically firm and irregular mass was evaluated by mammography and ultrasonography. On mammography, NE carcinomas appear as dense, irregular masses with spiculated or lobulated margins.(7,8) The same as was seen in our case. These tumors are seen on ultrasonography as hypoechoic solid masses with irregular shapes, ill-defined margins and enhanced vascularity.(1,7-9) In our case, the US findings included a heterogeneous (hypoechoic and hyperechoic) pattern with increased vascularity. Because the radiological features of NE carcinomas are nonspecific, therefore, fine needle aspiration or core needle biopsy is required for making the diagnosis.(10) We used core needle biopsy for making the preoperative diagnosis and the tumor was easily diagnosed the NE carcinoma. The histology of the mastectomy specimen, was consistent with the well known characteristics of NE carcinoma. Solid nests or sheets that consist of a uniform cell population, nuclear palisading and pseudorosette formation are the distinctive histologic findings in NE neoplasms.(11) All of these findings were observed in the present case. Oval and polygonal tumor cells with hyperchromatic nuclei and scant cytoplasm were disposed in solid-sheet like trabeculles. The mitotic index of the cells was low. This tumor pattern might be considered a solid papillary type as described Papotti et al.(4) In addition to its clinicopathological features, NE carcinoma of the breast may show the expression of NE markers such as chromogranin and synaptophysin.(12) According to Sapino and Bussolati, only tumors that can effectively express chromogranin and synaptophysin should be considered NE carcinomas of the breast.(13) However, there has been no consistently reported pattern of immunoreactivity for these markers. In the present case, the tumor was positive for chromogranin A and synaptophysin in more than 50% of cells, and so this supports the diagnosis. Also, the tumor in our patient was positive for ER/PR but it was negative for a c-erb-B2 expression. This immunoreactive profile can even be suggestive of primary NE carcinoma of the breast, as emphasized by other studies.(7,14) A small tumor diameter positivity for ER/PR receptor, and reduced Ki-67 activity were the good parameters for the prognosis.(2,13) Nonetheless, it is essential to confirm that the breast tumor has not metastasized from elsewhere. Thus, we performed further studies, including CT of the thorax and abdomen, an ocreotide scan and bone scintigraphy, and we ruled out any nonmammary site of tumor or any metastases from the breast to any other site of the body.
A standard treatment protocol is still lack for these uncommon carcinomas. However, their similar morphology, clinical behavior and histology to adenocarcinoma can make reasonable that these neoplasms should be treated like adenocarcinoma of the breast. Recent reports have shown a more favorable prognosis when these tumors are detected at an early stage.(15) Our patient who had a relatively early stage tumor underwent a modified radical mastectomy due to the tumor's multifocal appearance on mammography. Sentinel lymph node sampling wasn't performed because of the palpable suspicious lymph nodes metastasis. There is no definitive information in the literature about the sentinel lymph node sampling for neuroendocrine tumors of the breast. No adjuvant chemotheraphy was prescribed because of the patient's advanced age, and negative axillary lymph node status. Instead, hormonotheraphy with tamoxifen was given. The patient is alive without recurrence for 12 months after surgery. The short follow up period limits us to state the true prognosis. Although an earlier tumor stage, and hormone receptor sensitivity seem to be related to a good prognosis, large series with a longer follow-up periods are required to understand the actual behaviors of these tumors. Although breast neuroendocrine tumors are rare, those patients who are suspected of having these tumor should be throughly examined for metastases and pathological examinations should be carefully done.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download