Abstract
Purpose
The aim of this study was to determine the incremental effectiveness (the differences in progression-free survival between treatments), the incremental cost and the incremental cost-effectiveness of Genexol-PM compared to Paclitaxel when these drugs were used as treatment for patients with metastatic breast cancer.
Methods
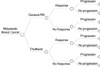
In the absence of any comparative direct evidence of the relative efficacy of Paclitaxel and Genexol-PM in this setting, a meta-analysis was conducted to determine the effects of the Paclitaxel on the health outcome. The decision tree model was constructed to evaluate the two treatment regimens. All the costs are in 2008 Korean Won (KW) and they were evaluated according to the 3rd party payer perspective, and the direct nonmedical and indirect costs were excluded.
Results
When compared with Paclitaxel, Genexol-PM was shown to increase the response rate and the time to progression for patients with metastatic breast cancer. Although the overall treatment costs of Genexol-PM were slightly higher than those of Paclitaxel, Genexol-PM was associated with a delayed time to progression of 4.78 months per patient. The incremental cost effectiveness ratio for Genexol-PM versus Paclitaxel was KW 2,295,228 per year gained, which is far below the per capita GDP or the threshold of the willingness-to-pay in Korea.
Figures and Tables
 | Figure 2Tornado diagram (cost-effectiveness threshold: 20,000,000 Korean Won).
M=Million Korean Won.
|
References
1. Kim SW, Han W, Jeong J, Park HK, Noh WC, Lee ES, et al. The policy proposal for effective prevention and management of breast cancer. J Breast Cancer. 2006. 9:270–292.

2. SEER Cancer Statistics Review, 1975-2004. National Cancer Institute. 2007. accessed September 24th, 2009.
http://seer.cancer.gov/csr/1975_2004.
3. 2005 Annual report of the central cancer registry in Korea. accessed September 24th, 2009. Ministry for Health Welfare and Family Affairs, Cancer Registry Center;
http://www.cancer.go.kr/.
4. Park WC, You YK, Choi SH, Suh YJ, Oh SC, Cho WI, et al. Therapeutic effects of paclitaxel (Taxol) in metastatic breast cancer. J Korean Surg Soc. 1999. 56:509–514.
5. Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, et al. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008. 108:241–250.

6. Brown RE, Hutton J, Burrell A. Cost-effectiveness of treatment options in advanced breast cancer in the UK. PharmacoEconomics. 2001. 19:1091–1102.

7. Launois R, Reboul-Marty J, Henry B, Bonneterre J. A cost-utility analysis of second-line chemotherapy in metastatic breast cancer. Docetaxel versus paclitaxel versus vinorelbine. Pharmacoeconomics. 1996. 10:504–521.

8. Limwattananon S, Limwattananon C, Maoleekulpairoj S, Soparatanapaisal N. Cost-effectiveness analysis of sequential paclitaxel adjuvant chemotherapy for patients with node positive primary breast cancer. J Med Assoc Thai. 2006. 89:690–698.
9. Holmes FA, Walters RS, Theriault RL, Forman AD, Newton LK, Raber MN, et al. Phase II trial of taxol, an active drug in the treatment of metastatic breast cancer. J Natl Cancer Inst. 1991. 83:1797–1805.

10. Reichman BS, Seidman AD, Crown JPA, Heelon R, Hakes TB, Lebwohl DE, et al. Paclitaxel and recombinant human granulocyte colonystimulating factor as initial chemotherapy for metastatic breast cancer. J Clin Oncol. 1993. 11:1943–1951.

11. Davidson NG. Single-agent paclitaxel at first relapse following adjuvant chemotherapy for breast cancer. Semin Oncol. 1995. 22:2–6.
12. Dieras V, Marty M, Tubiana N, Corette L, Morvan F, Serin D, et al. Phase II randomized study of paclitaxel versus mitomycin in advanced breast cancer. Semin Oncol. 1995. 22:33–39.
13. Seidman AD, Tiersten A, Hudis C, Gollub M, Barrett S, Yao TJ, et al. Phase II trial of paclitaxel by 3-hour infusion as initial and salvage chemotherapy for metastatic breast cancer. J Clin Oncol. 1995. 13:2575–2581.

14. Davidson NG, Chick JB, Perren TJ, Campbell N, Thompson JM, Chan YT. A phase II study of single agent paclitaxel in patients at first relapse following initial chemotherapy for breast cancer. Clin Oncol. 1996. 8:358–362.

15. Fountzilas G, Athanassiades A, Giannakakis T, Bafaloukos D, Karakousis K, Dombros N, et al. A phase II study of paclitaxel in advanced breast cancer resistant to anthracyclines. Eur J Cancer. 1996. 32:47–51.

16. Nabholtz JM, Gelmon K, Bontenbal M, Spielmann M, Catimel G, Conte P, et al. Multicenter, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. J Clin Oncol. 1996. 14:1858–1867.

17. Hudis C, Riccio L, Holmes F, Seidman A, Baselga J, Currie V, et al. Phase II study of semisynthetic paclitaxel in metastatic breast cancer. Eur J Cancer. 1997. 33:2198–2202.

18. Michael M, Bishop JF, Levi JA, Bell DR, Zalcberg JR, Friedlander ML, et al. Australian multicentre phase II trial of paclitaxel in women with metastatic breast cancer and prior chemotherapy. Med J Aust. 1997. 166:520–523.

19. Geyer CE Jr, Green SJ, Moinpour CM, O'Sullivan J, Goodwin DK, Canfield VA, et al. Expanded phase II trial of paclitaxel in metastatic breast cancer: a Southwest Oncology Group study. Breast Cancer Res Treat. 1998. 51:169–181.

20. Ito Y, Horikoshi N, Watanabe T, Sasaki Y, Tominaga T, Okawa T, et al. Phase II study of paclitaxel (BMS-181339) intravenously infused over 3 hours for advanced or metastatic breast cancer in Japan. BMS-181339 Breast Cancer Study Group. Invest New Drugs. 1998. 16:183–190.
21. Bishop J, Dewar J, Toner G, Smith J, Tattersall M, Olver I, et al. Initial paclitaxel improves outcome compared with CMFP combination chemotherapy as front-line therapy in untreated metastatic breast cancer. J Clin Oncol. 1999. 17:2355–2364.

22. Gelmon K, Eisenhauer E, Bryce C, Tolcher A, Mayer L, Tomlinson E, et al. Randomized phase II study of high-dose paclitaxel with or without amifostine in patients with metastatic breast cancer. J Clin Oncol. 1999. 17:3038–3047.

23. Paridaens R, Biganzoli L, Bruning P, Klijn JG, Gamucci T, Houston S, et al. Paclitaxel versus doxorubicin as first-line single-agent chemotherapy for metastatic breast cancer: a European Organization for Research and Treatment of Cancer Randomized Study with cross-over. J Clin Oncol. 2000. 18:724–733.

24. Rivera E, Holmes FA, Frye D, Valero V, Theriault RL, Booser D, et al. Phase II study of paclitaxel in patients with metastatic breast carcinoma refractory to standard chemotherapy. Cancer. 2000. 89:2195–2201.

25. Chao TC, Chu Z, Tseng LM, Chiou TJ, Hsieh RK, Wang WS, et al. Paclitaxel in a novel formulation containing less Cremophor EL as first-line therapy for advanced breast cancer: a phase II trial. Invest New Drugs. 2005. 23:171–177.

26. Liu F, Jiang ZF, Song ST, Liu XQ, Wang T, Yan M, et al. Relation of dose intensity and efficacy, toxicity in paclitaxel as a single agent for advanced breast cancer. Zhonghua Zhong Liu Za Zhi. 2005. 27:56–58.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download