Abstract
Purpose
We wanted to evaluate the difference of the images and the clinicopathological characteristics of young-age female breast cancer patients as compared to older Korean women with breast cancer.
Methods
A total of 351 breast cancers cases during the previous 3 years were evaluated. A cut-off level of 40 years was used to divide the patients into the young (≤40 years, 86 cases, 24.5%) and older groups (>40 years, 265 cases, 75.5%). We reviewed the BI-RADS results, the sensitivity of mammography (MMG) and sonography (US), the presenting symptoms, the histopathological type, the post-operative stage and the receptor status. These factors were compared between the young age group and the older age group. Chi-squared tests were used for statistical analysis.
Results
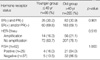
BI-RADS category 1 disease on the MMG (12.8% vs 6.4%, respectively) and BI-RADS category 3 disease on the US (3.5% vs 1.1%) were more common for the younger group as compared to the older group. The sensitivity of MMG and US was lower for the younger group than for the older group (69.2% and 82.3% vs 84.5% and 93.3%, respectively). Clinical symptom, histopathology, final stage, and the size of tumor or receptor status did not show statistical significant differences.
Although the incidence of breast cancer in Korea remains much lower than that in Western countries, it has been continuously increased. Recently, breast cancer has been reported highest incidence among the women's cancer in Korea. The breast cancer incidence reported in United States is 6.5% occurs by the age of 40 yr where as 11.3-17.3% of the incidence occurs in women younger than 35 yr of age according to the Korean Breast Cancer Society which is much higher.(1-3) Previous studies have shown mammography (MMG) to be ineffective as a diagnostic modality in young women, Ultrasound (US) would be the method of choice in the evaluation of young aged breast cancer.(4,5) However, there has not been a world consensus of US used as a screening tool for breast cancer. Also many reports suggested that in young age breast cancer is more aggressive and carries a higher mortality in younger women.(6-8) Whether a poorer prognosis is caused by a diagnostic delay or different characteristics of the tumor is still controversial. Most of the studies were limited to the young age cancer without comparison to old age group.(9-13) In this study, we evaluated the difference of imaging features on MMG and US, clinical and pathologic characteristics of young age breast cancer compared to older patients in Korean women.
Between March 2003 and December 2007, 351 patients who were treated for breast cancer were included in this study. We excluded patients who had not undergone preoperative assessment in our hospital or patients diagnosed of ductal carcinoma in situ. The average age of all patients with breast cancer was 48 yr (range 25-80 yr). All patients were grouped as young or old, using a cut-off level of 40 yr. The younger group (patients ≤40 yr) consisted of 86 patients (24.5%) with a mean age of 34.8 yr (range 25-40 yr). The older group (patients >40 yr) consisted of 265 patients (75.5%) with a mean age of 51.9 yr (range 41-80 yr).
All patients were examined with GE senographe 2000D for MMG and IU22 or HDI 2000 (Advanced Technology Laboratories, Bothell, USA) using a broad-bandwidth (7-12 MHz) linear probe for US.
All the results of MMG and US images were accessed according to the Breast Imaging Reporting and Data System (BI-RADS lexicon).(14) Assessment was made by two breast specialized radiologists. Each image has been discussed and consensus has come together between two radiologists reviewing the cases. At first, all the results of assessment, except for category 6, were compared between two groups. And then, they were reviewed to evaluate the diagnostic sensitivity of breast cancer in two groups. To evaluate the sensitivity, BI-RADS for MMG and US were categorized as benign (categories 1/2/3) and malignant (categories 4/5) from the assessed data. And the rest of the cases, category 0 and 6 on MMG and category 6 on US, were categorized after reassessment through retrospective image analysis as benign and malignant.
Presenting symptom, final histopathological type of breast cancer, post-operative stage, receptor status were reviewed retrospectively from the medical charts on computerized database system. For the evaluation of hormonal receptors, expression of estrogen receptor, progesterone receptor, were categorized as negative when measured less than 25% and as positive when measured more than 75%. HER-2 was considered positive when expressed 3 positive and additional FISH was carried out in 62 patients when HER-2 were under expressed.
Among 351 breast cancer cases, the results of BI-RADS category of MMG and US between young (n=86) and old (n=265) breast cancers are summarized in Table 1, 2. The frequency of incomplete assessed (Category 0) lesions was not different between young group (18.6%) than in old (19.2%) group. But young age breast cancer showed Category 1 in 12.8% in MMG which is about twice higher than old group (6.4%). On the other hand, Category 5 was about 1.5 times more in old group (46.1%) than the younger one (29.0%). There was no difference in proportion of Category 1 and 2 lesions (0% vs 1.5%) between two groups, but the frequency of Category 3 lesions were about 3 times much higher in young age group (3.5% vs 1.1%) than older one. On the contrary, Category 5 was about 1.5 times more in older group (62.7%) than the younger group (41.9%). The sensitivity of mammography for younger group was 69.2%, and 82.3% for US and for the older group sensitivity of mammography was 84.5% and that of US was 93.3%.
Approximately 23% of the patients had no specific symptoms and detected through the screening, and more than 60% of breast cancers were presented as palpable mass in both groups. Younger group had more complaints of pain (10.5% vs 3.0%) (p=0.035).
There was no statistical significance of histopathology of breast cancers between younger and old group. Younger group had almost six times higher frequency of mucinous carcinoma than old group (12.8% vs 2.3%). Pagets disease, papillary carcinoma and infiltrating lobular carcinoma were found in small number in older group in less than 3%.
Advanced post-operative tumor stage (stage III and IV) was not higher in young age group compare to the older group (15.1% vs 22.6%). For the hormonal receptor status, the frequency of estrogen and progesterone receptor status and HER-2 amplifications showed no clinical significant difference in old group compare with younger one.
The incidence of breast cancer reported in Korea is highest in women 50 yr of age and over,(3,15) which indicates that the average age is lower by 5-10 yr as compared to Western countries. This is the reason for Koreans to start screening mammography in earlier age of 40 compare to the western countries.(16) A cut off age group for breast cancer in young women is usually set at 35 yr of age, but was changed to 40 in our study, since it is the common starting age for screening mammography in Korea.(16,17) Despite many of the existing reports of worse prognostic factors and different biopathological profiles in young-age breast cancer patients as compare to the older counterparts, controversy still exists over this delay in diagnosis. So we concentrated on the analyzing the different imaging features of these two groups and also analyzed the clinicopahtological features.
In a study of MMG and US features in young breast cancer compare to older group, BI-RADS category 1 in MMG and Category 3 in US were more common in the younger group. Young aged breast cancer more often was shown as MMG negative than older group and the sensitivity was also lower compared to the older group. This result may be caused by background dense breast which mask the lesion depicted on MMG. Since the screening MMG is not recommended in patients younger than 40, most of patients who had taken MMG were symptomatic patients or referred patients from the local clinics but the role of pre-operative MMG in young aged patient has been controversial. In a study by Hindle et al.,(12) a retrospective review of 1,908 consecutive initial MMG reports of symptomatic women younger than 35 yr old were carried out and concluded that US was recommended by the radiologists in 37% of the study cases. So, routine initial MMG was not cost-effective or clinically beneficial in the evaluation of breast lesions. Most of the authors who studied the Method of choice imaging modalities for young symptomatic patients were thoroughly looked through breast US with careful reading of MMG.(4,18-21) Beyond identifying the lesion, previous reports described well-defined oval round masses may be one of the high incidence (42.7%) characteristics of the MMG findings in the young-age group in Asian women,(10) where as microcalcifications (57%) were the single most common finding in western country.(22,23) Our result also support this study in a way that MMG detected lesions were demonstrated as oval round masses in US more frequently in young aged breast cancer patients. The lesions in the younger patients were more likely to be undetected or interpreted as benign, especially in women with dense breasts. MMG is a valuable imaging modality in young age breast cancer patients with presenting symptoms, even though negatively appeared MMG was more frequent, and when a palpable mass is detected, a further workup with a suspicion of malignancy should be evaluated in younger aged group with further evaluation of US.
US features, categorized as probably benign finding, were significantly higher in younger patient. Although, approximately 3% in young group presented as well-defined oval or round nodules, which had a high possibility of mimicking a benign lesion, this results carries a limitation, a selection bias where biopsy were not carried out throughout all Category 3 patients but were selectively chosen so we do not know the actual number which would turn out to be malignant in Category 3 patients. We usually follow up category 3 patients within 6 months but when a patient feels fear for cancer or wants to be biopied, we would perform biopsy to confirm the pathology. In our result patients who were categorized as 3, their final pathology turned out to be mucinous carcinoma in 4 patients, infiltrating ductal carcinoma in 2 patients.
Many studies have shown that young age breast cancer, which is more aggressive and less responsive to treatment, may be caused by expression of an altered gene.(2,7,24,25) Higher frequencies of grade 3 tumors and estrogen receptor negativity contributed to worse prognosis.(26) For the hormonal receptor status, there was no difference in old group compare with younger one, a different finding from a higher proportion of receptor negativity in young age cancer as reported by Colleoni et al.(27) Others reported significantly higher T-stage and higher lymph node positivity and lower hormone receptor expression contributed poor outcome.(26) However, in our study, no statistical significant size differences, no stage or hormonal receptor status were noted.
The limitation of this study is that the comparison of patients aged ≤40 yr and >40 yr of age reflects patient referral and is not population-based. Also we did not sub-categorize the breast density which has meaningful implications when reviewing the images. We did not apply a strict Category 3 and Category 4 when performing the biopsy but was mostly depended upon the clinician referral so the results may be biased.
Breast cancers in young women were more frequently assessed as normal on MMG and probably benign on US, and less sensitive MMG and US than old women. Despite all the efforts trying to characterize the different clinical nd pathological differences between the groups, no statistically significant differences existed among various factors.
Figures and Tables
References
1. Hankey BF, Miller B, Curtis R, Kosary C. Trends in breast cancer in younger women in contrast to older women. J Natl Cancer Inst Monogr. 1994. (16):7–14.
2. Ahn SH, Hwang UK, Kwak BS, Yoon HS, Ku BK, Kang HJ, et al. Prevalence of BRCA1 and BRCA2 mutations in Korean breast cancer patients. J Korean Med Sci. 2004. 19:269–274.

3. Yoo KY, Kang D. Current researches on breast cancer epidemiology in Korea. Breast Cancer. 2003. 10:289–293.

4. Brand IR, Sapherson DA, Brown TS. Breast imaging in women under 35 with symptomatic breast disease. Br J Radiol. 1993. 66:394–397.

5. Houssami N, Irwig L, Loy C. Accuracy of combined breast imaging in young women. Breast. 2002. 11:36–40.

6. Sidoni A, Cavaliere A, Bellezza G, Scheibel M, Bucciarelli E. Breast cancer in young women: clinicopathological features and biological specificity. Breast. 2003. 12:247–250.

7. Chung M, Chang HR, Bland KI, Wanebo HJ. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer. 1996. 77:97–103.

8. Han W, Kim SW, Park IA, Kang D, Kim SW, Youn YK, et al. Young age: an independent risk factor for disease-free survival in women with operable breast cancer. BMC Cancer. 2004. 4:82.

9. Di Nubila B, Cassano E, Urban LA, Fedele P, Abbate F, Maisonneuve P, et al. Radiological features and pathological-biological correlations in 348 women with breast cancer under 35 years old. Breast. 2006. 15:744–753.

10. Foo CS, Su D, Chong CK, Chng HC, Tay KH, Low SC, et al. Breast cancer in young Asian women: study on survival. ANZ J Surg. 2005. 75:566–572.

11. Hartley MC, McKinley BP, Rogers EA, Kalbaugh CA, Messich HS, Blackhurst DW, et al. Differential expression of prognostic factors and effect on survival in young (< or =40) breast cancer patients: a case-control study. Am Surg. 2006. 72:1189–1194.
12. Hindle WH, Davis L, Wright D. Clinical value of mammography for symptomatic women 35 years of age and younger. Am J Obstet Gynecol. 1999. 180:1484–1490.

13. Johnstone PA, Moore EM, Carrillo R, Goepfert CJ. Yield of mammography in selected patients age or < =30 years. Cancer. 2001. 91:1075–1078.

14. D'Orsi CJ. American College of Radiology. Illustrated breast imaging reporting and data system (illustrated BI-RADS). 1998. 3rd ed. Reston: American College of Radiology.
15. Yoo KY, Kang D, Park SK, Kim SU, Kim SU, Shin A, et al. Epidemiology of breast cancer in Korea: occurrence, high-risk groups, and prevention. J Korean Med Sci. 2002. 17:1–6.

16. Lee SY, Jeong SH, Kim J, Jung SH, Song KB, Nam CM. Scheduling mammography screening for the early detection of breast cancer in Korean women. J Med Screen. 2007. 14:205–209.

17. Liberman L, Dershaw DD, Deutch BM, Thaler HT, Lippin BS. Screening mammography: value in women 35-39 years old. AJR Am J Roentgenol. 1993. 161:53–56.

18. Bassett LW, Ysrael M, Gold RH, Ysrael C. Usefulness of mammography and sonography in women less than 35 years of age. Radiology. 1991. 180:831–835.

19. Cohen MI, Mintzer RA, Matthies HJ, Bernstein JR. Mammography in women less than 40 years of age. Surg Gynecol Obstet. 1985. 160:220–222.
20. Grumbach Y, Nguyen HT. Mammography in younger women. Curr Opin Radiol. 1991. 3:602–610.
21. Muttarak M, Pojchamarnwiputh S, Chaiwun B. Breast cancer in women under 40 years: preoperative detection by mammography. Ann Acad Med Singapore. 2003. 32:433–437.
22. Shaw de Paredes E, Marsteller LP, Eden BV. Breast cancers in women 35 years of age and younger: mammographic findings. Radiology. 1990. 177:117–119.

23. Houssami N, Irwig L, Simpson JM, McKessar M, Blome S, Noakes J. Sydney Breast Imaging Accuracy Study: comparative sensitivity and specificity of mammography and sonography in young women with symptoms. AJR Am J Roentgenol. 2003. 180:935–940.

24. Lee WY. Frequent loss of BRCA1 nuclear expression in young women with breast cancer: an immunohistochemical study from an area of low incidence but early onset. Appl Immunohistochem Mol Morphol. 2002. 10:310–315.

25. Golshan M, Miron A, Nixon AJ, Garber JE, Cash EP, Iglehart JD, et al. The prevalence of germline BRCA1 and BRCA2 mutations in young women with breast cancer undergoing breast-conservation therapy. Am J Surg. 2006. 192:58–62.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download