Abstract
Purpose
To assess clinical factors and volumetric parameters associated with clinically significant symptomatic radiation pneumonitis (RP), which requires steroid medication after radiotherapy (RT).
Methods
Medical records of 204 irradiated breast cancer patients were reviewed. Percent lung volume (PLV) receiving more than 20 Gy was measured from CT-based treatment plan and was correlated with the central lung distance (CLD) of local and regional fields. PLV was also evaluated as a predictive factor of symptomatic RP, along with other previously reported clinical factors.
Results
Average (±standard deviation) actual irradiated lung volume and PLV for breast/chest wall irradiation were 169 (±50.6) cm3 and 14.9 (±3.8)%, respectively. Addition of regional irradiation resulted in increase of 183 (±80.2) cm3 in actual irradiated lung volume and 16.5 (±6.2)% in PLV. The correlation between CLD of the local fields and PLV was significant, with 1 cm of CLD corresponding to approximately 6% of PLV. CLD of the regional field was also significantly associated with PLV: a CLD of 3 cm corresponds to a PLV of approximately 13%; a CLD of 4 cm, approximately 17%; and a CLD of 5 cm, approximately 21%. RP developed in 11 patients (5.4%). There was an increased incidence of RP among patients who underwent local RT vs local and regional RT (2.4% vs 12.1%, p=0.0192). In terms of PLV, total PLV ≥23% was associated with the development of RP (p=0.0467). Previously reported clinical factors failed to show statistically significant association.
Conclusion
Correlation between CLD and PLV for local and regional fields was significant on volumetric analysis. Although symptomatic RP requiring steroid medication was a rare complication, regional irradiation increased the incidence of RP, and such relationship can be expressed with a volumetric parameter of PLV.
Radiation pneumonitis (RP) is one of the most common complications after adjuvant radiotherapy (RT) for breast cancer. The incidence of clinically significant RP, defined as symptomatic pneumonitis requiring steroid medication, varies from 1% to 29%.(1,2) Several risk factors for RP have been evaluated, and the treated lung volume has been best studied.(1-4) Central lung distance (CLD) measured on 2-dimensional simulation film has been shown to estimate the irradiated lung volume.(5-7) This parameter helped to predict the development of RP. However, several reports failed to show the significant correlation between CLD and RP.(1,3,8) To date, no reliable volumetric variable to predict RP has been established in breast cancer, whereas several variables have been suggested in lung cancer.(9-11)
Computed tomography (CT) simulation using a dedicated CT simulator was performed for every breast cancer patient at our institution. By analyzing the dose-volume histograms, the percent lung volume receiving more than 20 Gy (PLV) was calculated and it was compared with CLD. Through this volumetric analysis, the predictive value of PLV on the development of RP in patients treated with adjuvant RT for breast cancer was evaluated. Previously reported clinical factors, such as smoking, underlying lung disease, chemotherapy, and hormonal therapy associated with RP were also evaluated.
Between July 2001 and September 2002, the medical records of 204 patients who received adjuvant RT for breast cancer with 2 or more follow-up visits over more than 6 months period were reviewed. One hundred and fifty-two patients underwent breast conserving surgery, and 52 patients underwent mastectomy. Of these, 174 patients received adjuvant systemic chemotherapy. One hundred and twenty patients received chemotherapy before RT, 34 patients concurrently with RT, 13 patients after RT, and 7 patients in a sandwich style. Chemotherapy regimen consisted of cyclophosphamide, methotrexate, and 5-fluorouracil for 36 patients; cyclophosphamide, doxorubicin, and 5-fluorouracil for 40 patients; doxorubicin and cyclophosphamide for 16 patients; and a taxane-containing regimen for 78 patients. Adjuvant hormonal therapy with tamoxifen was given to 132 patients.
All patients underwent planning CT in the treatment position (supine on the breast angle board [MedTec, Orange City, USA] with ipsilateral arm elevated). Using a CT simulator (AcQSim; Phillips Medical Systems, Best, the Netherlands), CT images were obtained at 1 cm interval. The CT images were directly transferred to the 3-dimensional treatment planning system (AcQPlan, version 4.2; Phillips Medical Systems).
Opposing tangential 6MV photon was used for local RT. RT dose to breast or chest wall was 50 Gy at 2 Gy per fraction (Figure 1). Tumor bed boost was supplemented up to 10 Gy in 5 fractions for patients who underwent breast conserving surgery, using single en-face electron beam. Seventy-four patients received regional RT to supraclavicular fossa and axilla, using anterior-oblique 6MV photon up to 50 Gy. No patients received a posterior axillary boost.
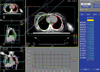
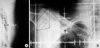
Simulation film taken from the conventional simulator was used to measure CLD. CLD of the local field was defined as the perpendicular distance from the posterior tangential field edge to the posterior part of the anterior chest wall (Figure 2A). CLD of the regional field was defined as the longitudinal distance from the matching line with tangents to the apex of the lung at the central axis (Figure 2B). PLV was defined as a lung volume receiving more than 20 Gy (V20), expressed in percent of the total volume of the ipsilateral lung. PLV was calculated from dose-volume histograms (Figure 1). Electron boost dose was not considered in the calculation of PLV.
Clinically significant RP was defined as symptomatic pneumonitis requiring steroid medication. Patients' medical records and medication prescriptions were reviewed. Steroid prescription for other reasons such as anti-emetic medication for on-going chemotherapy or symptomatic management for brain metastases and/or cord compression was censored.
The correlation between CLD and PLV was analyzed by two statistical methods: one-way ANOVA was used when CLD was considered a categorical variable and linear regression when CLD was considered a continuous variable. In both cases, PLV was considered a continuous variable. Fisher's exact test was used to analyze risk factors for RP. Multivariate analysis was performed using logistic regression analysis. All statistical analyses were done using SPSS software (release 12.0.1, SPSS Inc., Chicago, USA).
Average (±standard deviation) of measured total lung volume was 1,141 (±247) cm3. Irradiated actual lung volume and PLV of the local fields was 169 (±50.6) cm3 and 14.9 (±3.8)%, respectively. The PLV and CLD was significantly correlated (Table 1). Using the linear regression, the PLV of the local fields can be expressed as,
PLV (local)=1.14+5.86×CLD (local) (r=0.46, p<0.0001) (Figure 3A) where r is the regression coefficient.
When 74 patients who received regional irradiation were analyzed separately, addition of regional irradiation resulted in increase of 183 (±80.2) cm3 in actual irradiated lung volume and 16.5 (±6.2)% in PLV. Overall, irradiated actual lung volume and PLV in patients receiving regional irradiation was 349 (±100.9) cm3 and 31.2 (±7.3)%, respectively. The association between PLV and CLD was also statistically significant (Table 1). Using the linear regression, the PLV corresponding to the regional field can be expressed as,
PLV (regional)=-0.11+4.30×CLD (regional) (r=0.68, p<0.0001) (Figure 3B) where r is the regression coefficient.
There was an increased incidence of symptomatic RP among patients treated with local RT vs. local and regional RT (2.4% vs 12.1%, p=0.0192). When patients were grouped according to the total PLV, total PLV ≥23% was associated with the development of RP (p=0.0467). However, other patient-, tumor- and treatment-related factors failed to show the significant correlation with symptomatic RP (Table 2). On multivariate analysis incorporating the use of taxane, concurrent chemotherapy, and PLV, PLV was the only predictive factor for the development of RP (p=0.043).
CLD of the local field is a simple, but useful simulation parameter that predicts PLV. According to an early report from the Joint Center for Radiation Therapy, a CLD of 1.5 cm corresponds to a PLV of approximately 6%; a CLD of 2.5 cm, approximately 16%; and a CLD of 3.5 cm, approximately 26%.(5) Das et al.(6) also showed that PLV could be expressed as a function of CLD. This study reaffirmed these findings, but the correlation between CLD and PLV is rather weak (r=0.46). Breathing movement during field verification on the conventional simulator as well as set-up uncertainty might be responsible at least in part for the relatively weak correlation. However, our study may reflect the actual everyday clinical setting where various uncertainties are involved throughout the entire course of RT.
As for the PLV corresponding to the regional field, no useful predictor has been established yet. Bornstein et al.(5) only demonstrated that the mean value of PLV encompassed by the regional field was 12%, which was comparable to the PLV provided by the local field. Das et al.(6) noted a similar finding: 12% of ipsilateral lung was irradiated by the regional field on average, while 8.3% and 6.6% by the local field for left and right breasts, respectively. Given these observations, PLV by the regional irradiation is at least 50% of the total PLV when local and regional field is treated. Thus simulation parameter information from regional field in addition to that from local field would better predict PLV in this treatment setting. In the present study, CLD of the regional field was significantly associated with PLV: a CLD of 3 cm corresponds to a PLV of approximately 13%; a CLD of 4 cm, approximately 17%; and a CLD of 5 cm, approximately 21%.
Despite the correlation between CLD and PLV, increased CLD did not result in increased symptomatic RP in breast cancer patients.(1,3,8) As for lung cancer, on the contrary, the incidence of RP is proportional to the volume of treated lung.(9-11) Possible explanation for the negative results in breast cancer is the limited range of CLD of less than 3 cm in most patients, which would be of limited lung volume and this in turn leads to the low incidence of RP. Moreover, most studies analyzed only CLD of the local fields and did not consider the PLV provided by the regional fields. However, the PLV corresponding to the regional field accounts for at least 50% of the total PLV when local and regional field is treated, as mentioned previously. It should also be noted that regional irradiation increases the incidence of symptomatic RP, which reflects the contribution of the PLV by the regional irradiation on development of symptomatic RP.(1,3) Given these observations, we evaluated the predictive value of total PLV on the development of RP, and showed that total PLV ≥23% was associated with RP. Therefore, CLD of both local and regional field measured from 2-dimensional simulation film may provide more accurately estimated PLV and hence may better predict the risk of RP. Recently, Blom-Goldman et al.(12) suggested a PLV <30% was a reasonable dose-volume constraint for patients undergoing both local and regional RT. However, Allen et al.(4) failed to show any correlation between PLV and RP (p>0.5). Therefore, further studies are needed to confirm the predictive value of PLV on the development of RP.
There are limitations regarding dosimetric analysis. First, electron boost offered to patients after conserving surgery was not considered in the analysis. However, lung dose would not be altered after electron boost dose is added to the whole breast photon beam treatment when given factors are considered. First, opposing tangential beam arrangement was used for photon treatment, which would minimize dose gradient as shown in included case example. Second, en face beam arrangement with low energy electron (9-12 MeV) was used, which would not significantly alter lung dose past dose barrier made through tangential photon beam. It should also be noted that in this study only V20 was used to correlate CLD with PLV and then incidence of symptomatic RP. Significance of other dose bin has been reported for other thoracic malignancies, such as lung cancer and esophageal cancer. However, other dose bin values such as V15 or V10 would have limited additional meaning in our study. Because, unlike lung cancer or esophageal cancer treatment where multiple beams are used, opposing tangential field arrangement was used in analyzed breast cancer patients. Thus, variability for volume percentage at given dose bin was very limited. This could be readily confirmed through Figure 1, where dose-volume curve for treated lung is nearly a plateau from V5 to V30. In addition to PLV, other dose-volumetric parameters such as mean lung dose(13) and normal tissue complication probability(2) should be also evaluated to establish more reliable predictors of RP.
The role of taxane regimen in the development of RP is unclear. Taghian et al.(14) reported the increased incidence of RP in patients treated with paclitaxel. However, Yu et al.(15) noted much lower incidence when paclitaxel was given before anthracycline-based chemotherapy, suggesting the importance of time interval between paclitaxel and RT. In our study, the use of paclitaxel seemed to increase the development of RP with a marginal significance. However, significance of paclitaxel use on development of RP was lost after multivariate analysis. Concomitant chemotherapy other than taxane regimen was reported to be not related to increased incidence of RP,(16) and our study reaffirmed these findings.
Symptomatic RP requiring steroid medication was a rare complication after adjuvant RT for breast cancer. But, correlation between CLD and PLV for local and regional fields showed significance with volumetric analysis. Furthermore, addition of regional irradiation increased the incidence of RP, and such a relationship can be expressed with a volumetric parameter of PLV.
Figures and Tables
References
1. Lingos TI, Recht A, Vicini F, Abner A, Silver B, Harris JR. Radiation pneumonitis in breast cancer patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 1991. 21:355–360.

2. Gagliardi G, Bjöhle J, Lax I, Ottolenghi A, Eriksson F, Liedberg A, et al. Radiation pneumonitis after breast cancer irradiation: analysis of the complication probability using the relative seriality model. Int J Radiat Oncol Biol Phys. 2000. 46:373–381.

3. Lind PA, Marks LB, Hardenbergh PH, Clough R, Fan M, Hollis D, et al. Technical factors associated with radiation pneumonitis after local±regional radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2002. 52:137–143.

4. Allen AM, Prosnitz RG, Ten Haken RK, Normolle DP, Yu X, Zhou SM, et al. Body mass index predicts the incidence of radiation pneumonitis in breast cancer patients. Cancer J. 2005. 11:390–398.

5. Bornstein BA, Cheng CW, Rhodes LM, Rashid H, Stomper PC, Siddon RL, et al. Can simulation measurements be used to predict the irradiated lung volume in the tangential fields in patients treated for breast cancer? Int J Radiat Oncol Biol Phys. 1990. 18:181–187.

6. Das IJ, Cheng EC, Freedman G, Fowble B. Lung and heart dose volume analyses with CT simulator in radiation treatment of breast cancer. Int J Radiat Oncol Biol Phys. 1998. 42:11–19.

7. Neal AJ, Yarnold JR. Estimating the volume of lung irradiated during tangential breast irradiation using the central lung distance. Br J Radiol. 1995. 68:1004–1008.

8. Minor GI, Yashar CM, Spanos WJ Jr, Jose BO, Silverman CL, Carrascosa LA, et al. The relationship of radiation pneumonitis to treated lung volume in breast conservation therapy. Breast J. 2006. 12:48–52.

9. Kim TH, Cho KH, Pyo HR, Lee JS, Zo JI, Lee DH, et al. Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology. 2005. 235:208–215.

10. Graham MV, Purdy JA, Emami B, Harms W, Bosch W, Lockett MA, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 1999. 45:323–329.

11. Tsujino K, Hirota S, Endo M, Obayashi K, Kotani Y, Satouchi M, et al. Predictive value of dose-volume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2003. 55:110–115.

12. Blom-Goldman U, Svane G, Wennberg B, Lideståhl A, Lind PA. Quantitative assessment of lung density changes after 3-D radiotherapy for breast cancer. Acta Oncol. 2007. 46:187–193.

13. Kwa SL, Lebesque JV, Theuws JC, Marks LB, Munley MT, Bentel G, et al. Radiation pneumonitis as a function of mean lung dose: an analysis of pooled data of 540 patients. Int J Radiat Oncol Biol Phys. 1998. 42:1–9.

14. Taghian AG, Assaad SI, Niemierko A, Kuter I, Younger J, Schoenthaler R, et al. Risk of pneumonitis in breast cancer patients treated with radiation therapy and combination chemotherapy with paclitaxel. J Natl Cancer Inst. 2001. 93:1806–1811.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download