This article has been corrected. See "ERRATUM" in Volume 12 on page 123.
Abstract
Purpose
We have investigated the prognostic significance of the expression of basal markers for triple-negative (estrogen receptor-negative, progesterone receptor-negative and human epidermal growth factor receptor-2-negative) breast cancers (TNBCs).
Methods
An immunohistochemical study was performed on tissue microarrays constructed with 643 invasive breast carcinoma samples. We subclassified the TNBCs into basal phenotype (BP) and non-BP groups by the use of four different criteria according to the immunprofiles for cytokeratin5/6 (CK5/6), epidermal growth factor receptor (EGFR), vimentin, c-Kit, p63 and P-cadherin. The criteria consisted of criterion 1: CK5/6+ only, criterion 2: CK5/6+ and/or EGFR+, criterion 3: CK5/6+ and/or EGFR+ and/or vimentin+ and criterion 4: one or more marker(s) positive among the six basal markers. Each of these criteria, as well as the status of each individual marker, was evaluated to estimate prognosis for TNBC patients.
Results
Of the breast carcinomas, 165 cases (25.7%) were TNBCs. As compared with the non-TNBCs, TNBCs were associated with a larger tumor size (p=0.001), higher histological grade (p<0.001) and shorter overall survival (OS) (p=0.002) and disease-free survival (DFS) (p=0.05). Lymph node status, tumor size and expression of EGFR or c-Kit were independent prognostic factors for patients with TNBC. As compared with the non-BP, BP as defined by criterion 2 was an independent poor prognostic factor for OS and DFS among patients with a lymph node metastasis (p=0.044 and p=0.01) and among patients who received anthracycline-based adjuvant chemotherapy (p=0.009 and p=0.01, respectively).
Conclusion
Patients with TNBCs showed a poorer prognosis as compared to patients with non-TNBCs. Selected group of the basal-like breast cancers (BLBCs) defined by the immunohistochemical profiles of basal markers showed survival differences from non-BLBCs in subgroups of TNBCs with a homogeneous clinical finding.
Breast cancer is the most common malignant tumor found in women worldwide.(1) Among Korean women, breast cancer continues to increase in incidence, and it became the most common sporadic cancer identified in 2002,(2) although the age-standardized incidence rate per 100,000 Korean women is lower than the world average (26.2 vs 37.4).(1,3) Breast cancer is well known as a heterogeneous disease, with variable morphological and clinical features. In recent years, breast cancer has been classified into five subgroups according to gene expression profiles: luminal A, luminal B, normal breast-like, human epidermal growth factor receptor-2 (HER2)-overexpressing and basal-like.(4,5) These subgroups show significant differences in overall survival (OS); patients with basal-like and HER2-overexpressing cancers have the shorter survival times compared with hormone receptor positive cancers. Most basal-like breast cancers (BLBCs) are estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative (triple negative) at both transcriptional and translational levels.(6,7) Not only BLBCs but also normal breast-like cancers represent a triple negative (TN) phenotype.(8) Therefore, a triple negative breast cancer (TNBC) can be further classified into two groups, BLBC and non-BLBC. Many studies have defined a BLBC by immunohistochemical analysis because gene expression analysis at the mRNA (transcriptional) level has limitations when applied to formalin-fixed, paraffin-embedded tissue samples in a routine clinical setting. Expression of basal cytokeratins (CK5/6, CK14 and CK17),(7,9-26) epidermal growth factor receptor (EGFR),(6,7,19-24) c-kit,(23) p63,(13,19,25) and P-cadherin (19,25) has been used to define a BLBC in many studies. Recently, expression of vimentin,(7,21) laminin,(21) fascin,(22) caveolin-1 (27) and αB-crystallin (28) has emerged as promising markers for a BLBC. In addition to the expression of these basal markers, some investigators have required TN status, or ER-negative and HER2-negative status for defining a BLBC,(21,23-26) whereas other investigators did not consider hormone receptor and HER2 status.(9-18) The different definitions have resulted in diverse breast cancers that have been classified as BLBCs with different prognostic significance. Several studies have demonstrated that expression of basal cytokeratins is a poor prognostic factor.(11,14,18) However, when compared with ER-negative and non-BLBCs (9,26) or with grade-matched non-BLBCs,(16) a BLBC was not associated with a poorer prognosis.
TNBCs account for 11-32% of all invasive breast carcinomas.(29-34) Particular emphasis has been placed on TNBCs as there are limited treatment options for the cancers when compared with hormone receptor-positive or HER2-positive breast cancers and as they are closely related to a BLBC. There have been a few reports describing prognostic markers or therapeutic strategies for TNBCs.(32-34) In this study, we have evaluated expression of basal markers (CK5/6, EGFR, vimentin, c-kit, p63, and P-cadherin) in TNBCs by immunohistochemistry to investigate the prognostic significance of the expression of basal markers and to know whether each selected subgroup of breast cancers by basal phenotypes (BPs) has clinical significance for TNBCs.
For this study, we included 690 patients with primary invasive breast carcinoma who were treated at Yeungnam University Hospital between January 1987 and December 2003. We constructed tissue microarrays (TMAs) in our laboratory using a dermal punch biopsy needle with a 2.0 mm diameter. Two cores were taken from each tumor. Immunohistochemical screening for ER, PR, and HER2 was performed on the TMA slides. Finally, we could analyze 643 cases because of tissue loss during processing. Among them, 165 (25.7%) cases were TNBCs. All patients with a TNBC received axillary node dissection during surgery, and 154 (93.3%) out of 165 patients with a TNBC received adjuvant chemotherapy, including 95 patients that received anthracycline-based chemotherapy (5-fluorouracil and cyclophosphamide and either epirubicin or adriamycin). The median follow-up period of the patients was 66 months (range, 6-230 months). The patient age at initial diagnosis, tumor size, histological grade,(35) status of a lymph node metastasis, presence or absence of vascular invasion, local recurrence and presence of a distant metastasis were obtained from medical records and pathology reports. This study was approved by the Institutional Review Board of the Yeungnam University Hospital.
Immunohistochemical staining for ER, PR, HER2, CK5/6, EGFR, vimentin, c-kit, p63 and P-cadherin was performed on TMA sections. Briefly, 4 µm-thick tissue sections were cut from the TMA blocks and were mounted on poly-L-lysine-coated slides. Sections were deparaffinized in xylene and were then hydrated in a graded alcohol series. Heat-induced epitope retrieval was performed in a microwave oven for 12 min or with an autoclave for 10 min in ethylene diamine tetraacetic acid buffer (pH 8.0) or sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was inactivated by incubation of the sections in 4% H2O2 for 5 min. After rinsing the sections in phosphate-buffered saline, the tissue sections were then incubated with primary antibodies (Table 1) for 60 min at room temperature, and then visualization of positive staining was achieved with use of a DAKO En-Vision Plus-HRP detection kit (DAKO, Carpinteria, USA) according to the manufacturer's instructions.
Tumors were considered positive for expression of ER and PR when nuclear reactivity was observed in ≥10% of tumor cells at any intensity. Expression of HER2 was judged based on a staining score range of 0-3 by use of the Herceptest score and cases with a score of 3 were considered as positive.(36) The staining results for expression of CK5/6, EGFR, vimentin, c-Kit, p63 and P-cadherin were considered as positive when tumor cells stained undoubtedly for each marker in ≥1% of the tumor cells. Normal breast, colon, endometrium, liver, lung, prostate, spleen and stomach tissues incorporated into the tissue arrays were used as positive and negative controls for the immunohistochemical stains. Pulmonary carcinoma and gastrointestinal stromal tumor samples were used as positive controls for EGFR and c-Kit immunostains, respectively.
As there is no consensus for defining a BLBC, we applied four different criteria to classify TNBCs into BLBC and non-BLBC subgroups. The criteria for a BLBC include the following: criterion 1, a TNBC with positive expression of CK5/6; criterion 2, a TNBC with positive expression of CK5/6 and/or EGFR; criterion 3, a TNBC with positive expression of CK5/6 and/or EGFR and/or vimentin; criterion 4, a TNBC with positive expression of one or more marker(s) among CK5/6, EGFR, vimentin, c-Kit, p63 and P-cadherin. These BLBCs and non-BLBCs classified by each criterion were then compared in relation to the patient survival.
Statistical analysis was performed using SPSS 13.0 statistical software. The chi-squared test was used to evaluate correlations between TN status and the clinicopathological parameters in whole breast cancers. The mean age at diagnosis between TNBC and non-TNBC patients was compared using the t test. Survival analyses were conducted for OS and disease-free survival (DFS). DFS was defined as time to any type of recurrence, distant metastasis, or death relating with breast cancer. The survival difference between patients with a TNBC and a non-TNBC was analyzed using the log-rank test and survival curve was calculated using the Kaplan-Meier method. The survival differences between patients with a BLBC and a non-BLBC were calculated with the Cox proportional hazards model. Multivariate analysis with Cox proportional hazards regression was also used to evaluate any independent effect of prognostic factors on OS and DFS. A p-value of <0.05 was considered to indicate statistical significance.
Table 2 shows the main features of the TNBCs as compared with the non-TNBCs concerning different clinicopathological parameters and immunohistochemical results. The TNBCs showed a larger tumor size, higher histological grade and more frequent disease relapse (presence of local recurrence or distant metastasis) than the non-TNBCs. The frequencies of lymph node metastasis and vascular invasion were lower in the TNBCs than in the non-TNBCs, but the differences were not statistically significant. No differences were found between patients with TNBCs and non-TNBCs in relation to age and stage. Patients with a TNBC had reduced OS (p=0.002, Figure 1) and DFS (p=0.05). About 94% (30/32) of total deaths have occurred among patients with a TNBC within the first 6 yr after the diagnosis, while 72% (28/39) of total deaths have occurred among patients with a non-TNBC in the same period.
Basal markers that were rarely expressed in non-TNBCs were frequently expressed in TNBCs (Figure 2). Positive staining for CK5/6 in 38.2% (63/165) cases, EGFR in 21.2% (35/165) cases, vimentin in 30.3% (50/165) cases, c-Kit in 14.6% (24/164) cases, p63 in 9.1% (15/165) cases and P-cadherin in 46.1% (76/165) cases for the TNBC samples was determined. For the non-TNBC samples, positive staining for CK5/6 in 0.6% (3/478) cases, EGFR in 1.0% (5/478) cases, vimentin in 2.7% (13/478) cases, c-Kit in 2.1% (10/474) cases, p63 in 1.9% (9/476) cases and P-cadherin in 32.6% (156/478) cases was determined.
Based on univariate analysis, lymph node status, tumor size, and vascular invasion were significantly associated with OS and DFS in patients with TNBCs. Among the six basal markers, expression of EGFR and c-Kit were associated with shorter OS and DFS (Table 3). The use of multivariate analysis including lymph node status, tumor size, vascular invasion, and expression of EGFR and c-Kit showed that lymph node status and tumor size were independent prognostic factors for OS and DFS in TNBCs. Expression of c-Kit was associated with shorter OS and expression of EGFR was associated with shorter DFS on multivariate analysis in TNBCs (Table 4).
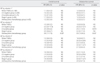
By criterion 1, 63 (38.2%) cases among 165 TNBCs were classified as BLBCs. Alternatively, 80 (48.5%) cases by criterion 2, 98 (59.4%) cases by criterion 3 and 131 (79.4%) cases by criterion 4 could be defined as BLBCs, respectively. We evaluated whether there is a prognostic difference between BP and non-BP groups in TNBC cases. We performed the same statistical analysis for four different populations of BP and non-BP groups that were generated by the four different criteria. The TNBCs were grouped based on stage (stage II group), lymph node status (lymph node negative and positive groups) and treatment option (patients who received anthracycline-based chemotherapy), and then further analyzed to determine if the BP has prognostic significance for each specified subgroup of TNBCs. We could not perform survival analysis in patients who did not receive chemotherapy due to limited number of patients. In univariate analyses, patients with a BP defined by any criterion did not show any difference in OS and DFS from patients with a non-BP in the entire population of TNBC patients. However patients with a BP defined by criterion 2 did show shorter DFS than patients with a non-BP in lymph node positive group and shorter OS and DFS in anthracycline chemotherapy group (Table 5). In multivariate analyses including tumor size, lymph node status, and BPs, the BP defined by criterion 2 was an independent poor prognostic factor for OS and DFS among patients with a lymph node metastasis (p=0.044 and p=0.01) and among patients who received anthracycline-based adjuvant chemotherapy (p=0.009 and p=0.01, respectively) (Table 4).
Molecular profiling of cancer using cDNA microarrays has led to a dramatic impact on the understanding of breast cancer. Among molecular subtypes defined by expression analysis, the BLBCs have recently received significant attention. As most BLBCs are ER-negative and HER2-negative, the term TNBC has previously been substituted for BLBC.(29) Although there is overlap between TNBCs and BLBCs, a TNBC is not synonymous with a BLBC.(8)
In our study, TNBCs were observed in 25.7% of invasive breast cancer cases. In another study performed in Korea, the frequency of TNBCs was 30.5%.(23) These frequencies were similar to those found in African-American women (24.6-31.6%)(24,31) and were slightly higher than the frequencies in Caucasian women (10.8-23%),(24,30,31,33) Japanese women (15%)(34) and Asian-Pacific Islanders living in the United States (11.7%).(31) This finding raises the possibility of a similarity in the manifestation of TNBC between Korean and African-American women.
TNBC had been found frequently in premenopausal women (<50 yr) in Western countries.(24,30-32) In our study, the mean ages at diagnosis were 46.7 yr and 47.3 yr for patients with TNBC and non-TNBC, respectively. The median age at diagnosis of invasive breast carcinoma is 46 yr in Korean women and age-specific incidences show peaks for patients in their forties and sharply decrease after that.(3) However, in Western countries, age-specific incidence rates increase sharply with age up to the time of menopause, and still increase at a slower rate after that age.(37) This international variation in age-specific incidence of breast cancer may explain why no age difference is observed between patients with a TNBC and non-TNBC in Korean women. There was also no age difference according to the basal marker expression in patients with a TNBC.
There are conflicting results on the prevalence of lymph node metastasis at the time of diagnosis in patients with a TNBC.(30,32) Recent studies have found no association between triple negativity and lymph node metastasis.(33,38) Our study showed lower frequencies of vascular invasion and lymph node metastasis in patients with a TNBC compared with patients with a non-TNBC. We believe that our findings are well matched with previous reports (9,10,15,23) that have shown lower lymph node positivity in BLBC as most BLBCs described in these studies were actually TNBCs.
From the point of view of a pathologist, the aggressiveness of TNBCs can be predicted by absence of hormone receptors, a larger tumor size and a high histological grade. Patients with a TNBC showed poorer survival than those with a non-TNBC, but the survival difference decreased over time. These results are similar to those of the previous studies (30,32) and suggest that patients with a TNBC have significantly shorter survival following the first metastatic event and that long term survivors with a TNBC may have comparable survival to patients with a non-TNBC.
While a BLBC was initially characterized by gene expression profiling, most recently published studies have used immunohistochemistry to define a BLBC in routine clinical samples (formalin-fixed and paraffin-embedded tissue). As there has been no international consensus on the immunohistochemical characterization of a BLBC, a wide variety of definitions have been used to classify BLBCs. Expression of various combinations of markers such as basal cytokeratins (CK5/6, CK14 and CK17), EGFR, c-Kit, p63, and P-cadherin have been used to define a BLBC.
Among these markers, the expression of at least one of the basal cytokeratins (CK 5/6, Ck14 or CK17) was the most commonly used definition of a BLBC. Therefore, at first, we classified TNBCs into BLBCs and non-BLBCs based only on basal cytokeratin expression (Criterion 1). We used CK5/6 because it was the most sensitive basal cytokeratin (15,23) and basal cytokeratins showed high concordance rates for staining among them.(34) The immunohistochemical panel proposed by Nielsen et al.(6) was ER-, HER2-, CK5/6 and/or EGFR+ and had a specificity of 100% and a sensitivity of 76% for detecting a BLBC defined by gene expression analysis. We tried this panel for identification of BLBCs (Criterion 2). Livasy et al.(7) observed the immunophenotypic profile of 18 breast carcinomas that had been defined as a BLBC by gene expression analysis. They reported that the most consistent immunophenotype for BLBC is ER-, HER2-, vimentin+, EGFR+, CK8/18+ and CK5/6+. Criterion 3 in our study was derived from their results. Criterion 4 reflects all basal markers that have been used to define BLBC in other studies. We determined cutoff points for positive expression of basal markers as at least ≥1% of tumor cells positive as this is the level of positive staining that has been used previously in many published reports.(6,18,21-24,26)
Many investigators did not consider hormone receptor status or HER2 status in selecting a BLBC or BP.(9-15,17-19) The number of markers used can affect the frequency of a BLBC and the particular markers used can result in selection of a different group of breast cancers. Therefore, BLBCs classified in many other studies are likely to be heterogeneous groups of breast cancer rather than a distinctive subgroup with a homogeneous immunophenotype. We considered TN status as a prerequisite for defining a BLBC because gene expression analysis and immunohistochemical profiles have shown that most BLBCs were ER- negative and HER2- negative.(6,7) Therefore, we compared BLBCs with non-BLBCs within all of the TNBCs in relation to patients' survival to exclude influences of hormone receptor and HER2 on tumor characteristics. In our results, patients with a BP defined by criterion 2 had shorter OS and DFS than patients with a non-BP among patients treated with anthracycline-based adjuvant chemotherapy and patients with positive lymph node(s). The poor prognosis of the selected group with BP is conferred almost entirely by the subset of tumors that are positive for EGFR as it was associated with patients' survival in the univariate analysis.
Recently, Rakha et al.(33) reported that BP is the only independent prognostic marker in node-negative TNBC group. This contrasts with our result because we have found prognostic significance of BP in the node-positive TNBCs. These contradictory results with regard to the prognosis of BP may be caused by different immunohistochemical definitions (CK5/6+ and/or CK14+ in Rakha's study and CK5/6+ and/or EGFR+ in our study) and selection bias of the cases. In addition, both studies did not show strong correlations between BP and prognosis (for DFS, p=0.02 in Rakha's study and p=0.01 in our study).
BLBC and TNBC are known to respond better to chemotherapy than non-BLBC and non-TNBC.(11,29) The influence of chemotherapy on the survival of patients with a BLBC has been evaluated in several studies. Some of the studies showed no survival difference between BLBCs and non-BLBCs among patients received adjuvant chemotherapy,(13,22) but other studies showed shorter OS and DFS in BLBC patients as compared with non-BLBC patients who received adjuvant chemotherapy.(14) In our study, TNBCs expressing CK5/6 and/or EGFR exhibited less benefit from anthracycline-based adjuvant chemotherapy and this result is concordant with a recent study.(39) As we defined BLBC with a more specific definition and specified chemotherapy regimens, our results suggest that selected group of BP may predict response of anthracycline-based adjuvant chemotherapy in patients with a TNBC. Poor outcome in patients with a TNBC expressing EGFR or c-Kit in our study suggests that EGFR and c-Kit could be therapeutic targets for these patients. However, further studies are needed to validate our result because the statistical significance is marginal.
Our results revealed that patients with TNBCs have poorer prognosis than patients with non-TNBCs and there is a significant degree of heterogeneity in BLBC as defined by immunohistochemical analysis of basal markers. Selected group of BLBCs by immunohistochemical profiles showed survival differences from non-BLBCs in subgroups of TNBC with a homogeneous clinical background. The use of basal markers in routine clinical practice can identify a subgroup of TNBCs with aggressive behavior and predict response of anthracycline-based chemotherapy.
Figures and Tables
 | Figure 1Kaplan-Meier survival curves of patients with TNBCs (n=165) and non-TNBCs (n=478). Patients with a TNBC had significantly reduced OS compared to those with a non-TNBC, especially within the first 6 yr.
TNBC=triple negative breast cancer; OS=overall survival.
|
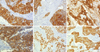 | Figure 2Representative immunohistochemical results. Some tumors showed strong and diffuse, cytoplasmic or membranous staining for (A) cytokeratin5/6 and (B) EGFR. (C) Vimentin was expressed in both tumor cells and stroma cells in some cases. (D) c-Kit immunoreactivity was observed in tumor cell cytoplasm. (E) p63 immunostaining was observed in tumor cell nuclei in a minority of cases. (F) P-cadherin immunoreactivity was characterized by strong membranous staining. Original magnification, ×200.
EGFR=epidermal growth factor receptor.
|
Table 3
Univariate analysis of patient outcome according to the clinicopathological parameters and basal marker expression in the TNBC patients

Table 4
Multivariate Cox regression analysis of factors associated with overall survival and disease-free survival in the TNBC patients
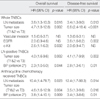
Table 5
Univariate analysis of patient outcome according to the basal phenotypes in different patients' groups
BP=basal phenotype; TNBC=triple-negative breast cancer; LN=lymph node; HR=hazard ratio; CI=confidence interval; NS=not significant.
*Criterion 1, TNBC expressing CK5/6; †Criterion 2, TNBC expressing CK5/6 and/or EGFR; ‡Criterion 3, TNBC expressing CK5/6 and/or EGFR and/or vimentin; §Criterion 4, TNBC expressing one or more markers among CK5/6, EGFR, vimentin, c-kit, p63 and P-cadherin.
References
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.

2. Shin HR, Jung KW, Won YJ, Park JG. 139 KCCR-affiliated Hospitals. 2002 Annual report of the Korea Central Cancer Registry: based on registered data from 139 hospitals. Cancer Res Treat. 2004. 36:103–114.

3. Lee JH, Yim SH, Won YJ, Jung KW, Son BH, Lee HD, et al. Population-based breast cancer statistics in Korea during 1993-2002: incidence, mortality, and survival. J Korean Med Sci. 2007. 22:Suppl. S11–S16.

4. Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000. 406:747–752.

5. Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001. 98:10869–10874.

6. Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004. 10:5367–5374.

7. Livasy CA, Karaca G, Nanda R, Tretiakova MS, Olopade OI, Moore DT, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod Pathol. 2006. 19:264–271.

8. Rakha EA, Tan DS, Foulkes WD, Ellis IO, Tutt A, Nielsen TO, et al. Are triple negative tumours and basal-like breast cancer synonymous? Breast Cancer Res. 2007. 9:404.

9. Jumppanen M, Gruvberger-Saal S, Kauraniemi P, Tanner M, Bendahl PO, Lundin M, et al. Basal-like phenotype is not associated with patient survival in estrogen-receptor-negative breast cancers. Breast Cancer Res. 2007. 9:R16.

10. Fulford LG, Reis-Filho JS, Ryder K, Jones C, Gillett CE, Hanby A, et al. Basal-like grade III invasive ductal carcinoma of the breast: patterns of metastasis and long-term survival. Breast Cancer Res. 2007. 9:R4.

11. Rakha EA, El-Rehim DA, Paish C, Green AR, Lee AH, Robertson JF, et al. Basal phenotype identifies a poor prognostic subgroup of breast cancer of clinical importance. Eur J Cancer. 2006. 42:3149–3156.

12. Turner NC, Reis-Filho JS, Russell AM, Springall RJ, Ryder K, Steele D, et al. BRCA1 dysfunction in sporadic basal-like breast cancer. Oncogene. 2007. 26:2126–2132.

13. Laakso M, Tanner M, Nilsson J, Wiklund T, Erikstein B, Kellokumpu-Lehtinen P, et al. Basoluminal carcinoma: a new biologically and prognostically distinct entity between basal and luminal breast cancer. Clin Cancer Res. 2006. 12:4185–4191.

14. Banerjee S, Reis-Filho JS, Ashley S, Steele D, Ashworth A, Lakhani SR, et al. Basal-like breast carcinomas: clinical outcome and response to chemotherapy. J Clin Pathol. 2006. 59:729–735.

15. Rakha EA, Putti TC, Abd El-Rehim DM, Paish C, Green AR, Powe DG, et al. Morphological and immunophenotypic analysis of breast carcinomas with basal and myoepithelial differentiation. J Pathol. 2006. 208:495–506.

16. Fulford LG, Easton DF, Reis-Filho JS, Sofronis A, Gillett CE, Lakhani SR, et al. Specific morphological features predictive for the basal phenotype in grade 3 invasive ductal carcinoma of breast. Histopathology. 2006. 49:22–34.

17. Laakso M, Loman N, Borg A, Isola J. Cytokeratin 5/14-positive breast cancer: true basal phenotype confined to BRCA1 tumors. Mod Pathol. 2005. 18:1321–1328.

18. van de Rijn M, Perou CM, Tibshirani R, Haas P, Kallioniemi O, Kononen J, et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol. 2002. 161:1991–1996.

19. Paredes J, Lopes N, Milanezi F, Schmitt FC. P-cadherin and cytokeratin 5: useful adjunct markers to distinguish basal-like ductal carcinomas in situ. Virchows Arch. 2007. 450:73–80.

20. Livasy CA, Perou CM, Karaca G, Cowan DW, Maia D, Jackson S, et al. Identification of a basal-like subtype of breast ductal carcinoma in situ. Hum Pathol. 2007. 38:197–204.

21. Rodríguez-Pinilla SM, Sarrió D, Honrado E, Moreno-Bueno G, Hardisson D, Calero F, et al. Vimentin and laminin expression is associated with basal-like phenotype in both sporadic and BRCA1-associated breast carcinomas. J Clin Pathol. 2007. 60:1006–1012.

22. Rodríguez-Pinilla SM, Sarrió D, Honrado E, Hardisson D, Calero F, Benitez J, et al. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin Cancer Res. 2006. 12:1533–1539.

23. Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: a comparison with hormone receptor and HER2/neu-overexpressing phenotypes. Hum Pathol. 2006. 37:1217–1226.

24. Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006. 295:2492–2502.

25. Matos I, Dufloth R, Alvarenga M, Zeferino LC, Schmitt F. p63, cytokeratin 5, and P-cadherin: three molecular markers to distinguish basal phenotype in breast carcinomas. Virchows Arch. 2005. 447:688–694.

26. Potemski P, Kusinska R, Watala C, Pluciennik E, Bednarek AK, Kordek R. Prognostic relevance of basal cytokeratin expression in operable breast cancer. Oncology. 2005. 69:478–485.

27. Pinilla SM, Honrado E, Hardisson D, Benítez J, Palacios J. Caveolin-1 expression is associated with a basal-like phenotype in sporadic and hereditary breast cancer. Breast Cancer Res Treat. 2006. 99:85–90.

28. Sitterding SM, Wiseman WR, Schiller CL, Luan C, Chen F, Moyano JV, et al. αB-crystallin: a novel marker of invasive basal-like and metaplastic breast carcinomas. Ann Diagn Pathol. 2008. 12:33–40.

29. Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007. 13:2329–2334.

30. Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007. 13:4429–4434.

31. Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the socalled triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007. 109:1721–1728.

32. Tischkowitz M, Brunet JS, Bégin LR, Huntsman DG, Cheang MC, Akslen LA, et al. Use of immunohistochemical markers can refine prognosis in triple negative breast cancer. BMC Cancer. 2007. 7:134.

33. Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007. 109:25–32.

34. Sasa M, Bando Y, Takahashi M, Hirose T, Nagao T. Screening for basal marker expression is necessary for decision of therapeutic strategy for triple-negative breast cancer. J Surg Oncol. 2008. 97:30–34.

35. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991. 19:403–410.

36. Jacobs TW, Gown AM, Yaziji H, Barnes MJ, Schnitt SJ. Specificity of HercepTest in determining HER-2/neu status of breast cancers using the United States Food and Drug Administration-approved scoring system. J Clin Oncol. 1999. 17:1983–1987.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download