Abstract
Intracystic papillary carcinoma (IPC) of the breast is a rare entity, which can be associated with ductal carcinoma in situ (DCIS) or an invasive carcinoma. There have been only limited reports describing IPC in Korea and the incidence among breast cancer cases in Korea has not yet been reported. From a database of 7,109 breast cancer cases treated surgically at Samsung Medical Center, we could only identify four IPC cases (0.056%). We also found that there are some differences between the clinicopathological characteristics of our cases and the alleged characteristics of IPC as reported from Western countries, such as a relatively young age of onset, small tumor size and various expression levels of hormonal receptors. We suspect this very low incidence may be caused by a true rarity of IPC in women in Korea or may be due to a lack of clinical interest for IPC. Upon presentation of our experience with IPC, we suggest the diagnosis for this rare disease entity needs to be reappraised.
Intracystic papillary carcinoma (IPC) of the breast is a rare entity, known to be accounting for between 0.5% and 1% of all breast cancers.(1,2) Papillary lesions in the breast have a wide spectrum of lesions that include benign papilloma, papillary variants of ductal carcinoma in situ (DCIS), and invasive papillary carcinoma. Traditionally, IPC was considered to be a variant of DCIS, as a localized form of intraductal papillary carcinoma that exists in a cystically dilated duct.(2) Although it has been believed that IPC may exist as an isolated localized non-invasive carcinoma, being associated with DCIS or with invasive carcinoma,(3) recent studies have suggested that IPC should be considered as a low risk invasive carcinoma.(4)
We tried to find out the data of IPC of Korean women, but only five reports of a single case could be found through the web-based search of KoreaMed (http://www.koreamed.org) and the first report was in 1997 with a case of 80-yr-old female breast cancer.(5-9) This small amount of work which has been done in Korea may be due to a rarity of IPC in Korean women, but this also may be due to lack of clinical interests for IPC since it was mistaken as a variant of DCIS which has a good prognosis after treatment, resulting in rough discrimination between IPC and DCIS. Given that IPC is considered a low risk invasive carcinoma, this should be reevaluated as an independent disease entity other than DCIS. After review of the 14-yr surgical and pathological database of a single institute, we could find only four cases of IPC among 7,109 cases who underwent surgery for breast cancer. Thus, we report these cases with a review of the recent literature.
The surgical and pathological database of Samsung Medical Center from November 1994 to October 2008 was investigated to identify all patients with a diagnosis of IPC of the breast by pathologic report. Surprisingly, only four patients were identified, representing 0.056% of surgically treated patients with breast cancer during this period. We reviewed clinical histories and radiologic data, preoperative and postoperative pathologic findings, treatment strategies and follow-up data for these patients.
All were female patients and the range of age was from 40 to 82 (mean: 61.3) yr (Table 1). Regarding the initial symptoms, three patients presented with a palpable mass and remaining one patient complained of bloody nipple discharge. All patients had mammograms and ultrasonography and two patients had MRI prior to surgery to identify any possible hidden lesions in other quadrants. On mammograms, all tumors were demonstrated as sharply or partially circumscribed mass lesions. The ultrasonography showed complex cystic or multilobulated tumors (Figure 1). All four patients were classified in BIRADS category 4 at the initial diagnostic radiology examination and they had core needle biopsies. The preoperative tissue diagnoses from core biopsies were various among cases. In 2 patients, it was reported as an intraductal papillary carcinoma. For each of the other two patients, 'invasive carcinoma with focal papillary architecture and apocrine differentiation'and'chronic active mastitis with atypical cells'was reported respectively (Table 1).
Breast conservation and additional axillary procedures (axillary dissection in one patient and sentinel lymph node biopsy in 3 patients) were performed for all patients (Table 1). The reason of conventional axillary dissection for one patient was the presence of enlarged lymph nodes on physical examination. Clear margins without involvement of tumor cells were obtainable in all of the patients. Except for one patient who refused radiotherapy, all the other patients underwent adjuvant radiotherapy.
The pathologic characteristics of the patients are described in Table 2. In the gross description of the lesions, well-circumscribed cystic masses or cystic tumor with lobulated margin were identified, of which median size was 1.6 cm (range, 1.0-1.9 cm). No patients had lymphatic metastasis of tumor cells. The nuclear grades were intermediate to high. Immunohistochemical study for estrogen receptor (ER), progesterone receptor (PR) and C-erb-B2 oncoprotein was performed. Strong and diffuse immunoreactivity for ER and PR was found in two cases but not observed in the other two cases. C-erb-B2 oncoprotein expression on immunohistochemistry examination was observed in two patients, not seemed to have any relationship with hormonal receptor status. Intracystic papillary carcinoma was characterized by one or several nodules of papillary carcinoma surrounded by a characteristic thick fibrous capsule. Absence of myoepithelial cells (MECs) are helpful in differentiating intracystic papillary carcinoma from intraductal papillary carcinoma.(10) (Figure 2A) To determine the presence of invasiveness by staining of MECs, immunohistochemical examination for p63 expression was performed within and at the periphery of the tumors of any of the 4 cases of IPC (Figure 2B). MECs were not found in the core of the papillae and rarely observed at the periphery of the tumor nodules. Associated DCIS was found in only one case, and in the other three cases, there was no DCIS or invasive component observed around the IPC lesion.
Tamoxifen was prescribed in two patients who had a strong ER and PR positivity with immunohistochemistry examination. One of these patients was diagnosed as IPC in contralateral breast despite of 2-yr tamoxifen treatment due to previously diagnosed DCIS. The other two patients were ER- and PR-negative so that tamoxifen was not prescribed. Adjuvant chemotherapy was not indicated for all these patients (Table 3). Although the follow-up period is not that long (mean: 29 months, range: 2-86 months), all patients are alive without any evidence of disease recurrence.
Papillary lesions comprise a wide spectrum in terms of their clinical presentation, behavior, morphologic features and malignant potential. Kraus and Neubecker(11) established criteria to distinguish benign papillomas from papillary carcinomas. Subsequently, papillary carcinomas can be divided into invasive types and noninvasive types.(12) The further subdivision of noninvasive papillary carcinomas into 2 types was made by Carter et al.(1); the first one is a localized form commonly referred to as IPC (or encysted papillary carcinoma) and the second one is a diffuse form known as the papillary variant of DCIS.(1,2) The preoperative diagnosis of papillary cancer from a core needle biopsy is made for only 17% to 43% patients with the definitive diagnosis of this lesion.(12-14) We have generally adopted surgical excision as a standard policy for all papillary lesions diagnosed by image-guided biopsy because of the possible chance of a hidden malignancy.
Intracystic papillary carcinoma of the breast is a rare disease entity, which is most commonly encountered in elderly patients, mean age of 70-yr-old. It is usually located in the retroareolar or central area and it shows distinctive pathological features that must be distinguished from papillary variant of DCIS as well as invasive papillary carcinoma.(1,2) As mentioned above, the reports of IPC have been only a few in Korea, even including an IPC case report in a male patient.(5) In this study, only 4 patients were found from the database of 7,109 breast cancer patients treated surgically from November 1994 to October 2008. Since the incidence of IPC in Korean women has not been reported, this very low incidence of IPC in our institute may help to estimate the incidence of IPC among Korean women. However, it may be conceivable that this low incidence might have been caused by the minimal concern for IPC because of its relatively good prognosis after treatment, resulting in lack of interest to distinguish IPC from DCIS.
Traditionally, IPC has been considered an intraductal neoplasm, based upon the presumption from the gross and microscopic features that such papillary carcinomas are present within the structure of a cystic-dilated duct, unless they are associated with a definitely invasive component. However, recent evidences with immunohistochemical assay and reports about axillary and/or distant metastasis have raised the possibility that not all IPC lesions are in situ carcinomas.(4,15-17) The immunohistochemical assay for MEC with the specific markers (p63, smooth muscle myosin, caldesmon, calponin, etc.) is widely used to make the differential diagnosis of most invasive and noninvasive ductal tumors of the breast. This is demonstrated to be helpful in identifying the invasive characteristics of IPC since several reports have documented that IPC most commonly lack any MEC cell lining.(4,17) We also evaluated the presence and distribution of MEC with p63 and could find that p63 is not expressed at the core and periphery of tumors in our cases. We also evaluated the presence and distribution of MEC with p63 and could find that p63 is not expressed at the core and very rarely expressed in periphery of tumors in our cases. This paucity of p63 in our cases may represent the possibility of invasiveness of IPC. Some studies reported the axillary lymph node metastasis and distant metastasis of IPC with the range of 0% to 11% for axillary lymph node metastasis and 0% to 4% for distant metastasis.(2,3,17-19) The occasional finding of lymph node metastasis from invasive papillary carcinomas shows similar growth patterns of IPC, which has also been provided as an additional evidence for the invasive nature of IPC.(15) Considering these findings, the current consensus is that IPC may be a part of a spectrum of progression from in situ to invasive papillary carcinoma. For these reasons, the diagnosis of IPC has been reappraised. However, this recognition of IPC as an invasive carcinoma would not mean therapeutic changes because of the indolent clinical behavior of IPC.
We could find some differences between the characteristics of our cases, such as the relatively early age of onset especially in two patients and small sizes of tumor, and the previously known features of IPC (Table 1). Most reports have shown that IPC is a hormonal receptor positive tumor and drugs such as tamoxifen are to be considered as an adjuvant therapy for IPC. In this present study, the expression of hormonal receptor was identified in only half of the patients. Even in one patient, IPC was found during follow-up period with the use of tamoxifen after previous wide excision due to contralateral breast DCIS.
The treatment of IPC depends on the associated histologic findings. In the cases of pure IPC, complete local resection without axillary dissection is the treatment of choice. If DCIS or invasive component is present outside the main tumor, therapy has generally been changed according to the standard of treatment for non-IPC component of the breast. Lymph node metastasis is rare and the reported clinical course of IPC even with invasive component after adequate local treatment alone were excellent.(1,4) However, tumors with high nuclear grade, histologic grade and large surface area are more likely to metastasize to lymph node or recur locally.(2,19) In these cases, even in the case of pure IPC, especially those patients who are young, additional axillary approach like sentinel lymph node biopsy, adjuvant radiation and endocrine therapies should be considered.(13) Although adjacent foci of DCIS and/or invasive carcinoma can be found in about 40% to 70% of IPC patients,(2,3,18,19) most reports support local treatment alone as sufficient treatment for pure IPC.(13,19) Solorzano et al.(19) even reported that the incidence of recurrence of IPC did not differ between IPC only or with DCIS or invasive cancer, regardless of the type of surgery (local excision or mastectomy with or without lymph node dissection) and whether radiation was administered.
The prognosis for patients with IPC is excellent when treatment decisions are made according to associated pathology.(13) In a study with 77 patients, the 5- and 10-yr disease free survival rates were 96% and 91%, respectively.(3) In another report which followed 40 patients, disease-specific survival rate was 100% irrespective of the presence of combined DCIS or invasion.(19)
From the review of this small number of IPC cases, we suggest that the diagnosis of IPC needs to be reappraised in Korea, considering it has some characteristics between in situ and invasive papillary carcinoma. Although the incidence of IPC was very low among the breast cancer patients who were treated in our institute, it may be influenced by the lack of diagnostic interest for IPC. The incidence of IPC in Korean women needs to be further evaluated through a multicenter study with a pathologic standard for its diagnosis.
Figures and Tables
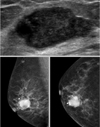 | Figure 1Mammography and Ultrasonography demonstrates a dense tumor with smooth and Iobulated contours which is the typical radiologic findings of intracystic papillary carcinoma. |
 | Figure 2(A) Photomicrograph of tumor reveals an intracystic papillary carcinoma with thick fibrous capsule (H&E, ×10). (B) Immnohistochemistry for myoepithelial cells (p63) is negative at the periphery as well as at the cores of the papillae (×200) (black arrow: the core of the papillae, blue arrow: the periphery of the tumor). |
References
1. Carter D, Orr SL, Merino MJ. Intracystic papillary carcinoma of the breast. After mastectomy, radiotherapy or excisional biopsy alone. Cancer. 1983. 52:14–19.

2. Leal C, Costa I, Fonseca D, Lopes P, Bento MJ, Lopes C. Intracystic (encysted) papillary carcinoma of the breast: a clinical, pathological, and immunohistochemical study. Hum Pathol. 1998. 29:1097–1104.

3. Lefkowitz M, Lefkowitz W, Wargotz ES. Intraductal (intracystic) papillary carcinoma of the breast and its variants: a clinicopathological study of 77 cases. Hum Pathol. 1994. 25:802–809.

4. Collins LC, Carlo VP, Hwang H, Barry TS, Gown AM, Schnitt SJ. Intracystic papillary carcinomas of the breast: a reevaluation using a panel of myoepithelial cell markers. Am J Surg Pathol. 2006. 30:1002–1007.

5. Yun SS, Choi SH, Kim SK, Park JK, Baek JM, Lee DH, et al. Intracystic papillary carcinoma in the male breast: a case report. J Breast Cancer. 2008. 11:151–155.

6. Woo OH, Yong HS, Kim A, Lee JB, Koo BH, Kang EY. Intracystic papillary carcinoma with extensive hemorrhage of the breast: sonographic and advanced MR findings: a case report. J Korean Radiol Soc. 2006. 55:511–514.

7. Park SC, Oh SJ, Kim KM, Kim JS, Jung SS. Case of an intracystic (encysted) papillary carcinoma of the breast. J Korean Surg Soc. 2000. 59:810–814.
8. Ko KH, Kim EK, Park BW. Invasive papillary carcinoma of the breast presenting as post-traumatic recurrent hemorrhagic cysts. Yonsei Med J. 2006. 47:575–577.

9. Lee AW, Choi YJ, Lee KY, Kim BK, Kim SM, Shim SI. Fine needle aspiration cytology of intracystic papillary carcinoma of the breast. Korean J Cytopathol. 1997. 8:179–184.
10. Collins LC, Schnitt SJ. Papillary lesions of the breast: selected diagnostic and management issues. Histopathology. 2008. 52:20–29.

11. Kraus FT, Neubecker RD. The differential diagnosis of papillary tumors of the breast. Cancer. 1962. 15:444–455.

12. Fisher ER, Palekar AS, Redmond C, Barton B, Fisher B. Pathologic findings from the National Surgical Adjuvant Breast Project (protocol no. 4). VI. Invasive papillary cancer. Am J Clin Pathol. 1980. 73:313–322.

13. Fayanju OM, Ritter J, Gillanders WE, Eberlein TJ, Dietz JR, Aft R, et al. Therapeutic management of intracystic papillary carcinoma of the breast: the roles of radiation and endocrine therapy. Am J Surg. 2007. 194:497–500.

14. Gendler LS, Feldman SM, Balassanian R, Riker MA, Frencher SK, Whelan DB, et al. Association of breast cancer with papillary lesions identified at percutaneous image-guided breast biopsy. Am J Surg. 2004. 188:365–370.

15. Viale G. Pathological definitions of invasion, metastatic potential and responsiveness to targeted therapies. Breast. 2007. 16:Suppl 2. S55–S58.

16. Mulligan AM, O'Malley FP. Metastatic potential of encapsulated (intracystic) papillary carcinoma of the breast: a report of 2 cases with axillary lymph node micrometastases. Int J Surg Pathol. 2007. 15:143–147.

17. Hill CB, Yeh IT. Myoepithelial cell staining patterns of papillary breast lesions: from intraductal papillomas to invasive papillary carcinomas. Am J Clin Pathol. 2005. 123:36–44.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download