Abstract
Purpose
We want to validate the use of the silver-enhanced in situ hybridization (SISH) technique as comparised with fluorescence in situ hybridization (FISH) technique for assessing the HER2 gene amplification of breast carcinoma.
Methods
Tissue microarray (TMA) blocks from 58 breast cancer specimens were prepared and the concordance between HER2 gene amplification in breast cancer was determined by the FISH (PathVysion®, Abbott/Vysis) technique and the automated silver in situ hybridization (SISH, INFORM™, Ventana) technique. For comparison, all the specimens were stained by immunohistochemistry (Ventana-PATHWAY®4B5). Evaluation was performed by two pathologists and with following the instructions of the manufacturers and the guidelines of the American Society of Clinical Oncology/College of American Pathologists.
Results
The results of SISH and FISH were identical in all 58 cases; 17 cases showed HER2 amplification, and on the other hand, 41 cases didn't show HER2 amplification. Five weakly positive (2+) cases in immunohistochemistry staining revealed one HER2 amplification and four no HER2 amplification on both SISH and FISH. The SISH results of the HER2/CEP17 ratio were well correlated with the FISH results of the HER2/CEP17 ratio (correlation coefficient r=0.745, Linear regression r2=0.555, p<0.001).
Conclusion
The results of the SISH technique for assessing the HER2 status of excised breast carcinoma is comparable to the result obtained by FISH. However, SISH has the advantage of having permanent end result that can be visualized by an ordinary light microscope and less laborious preparation and time is needed than is required by FISH. SISH seems to be more feasible than FISH for assessing HER2 amplification of breast cancer.
Figures and Tables
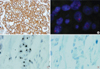 | Figure 1HER2 IHC, FISH and SISH results in breast carcinomas showed (A) the example of IHC of 3+ staining, (B) the example of HER2 amplification in FISH (HER/CEP17 ratio >2.2), (C, D) the example of HER2 amplification in SISH (C: HER2, D: CEP17, HER2/CEP17 ratio >2.2).
IHC=immunohistochemistry; SISH=silver in situ hybridization; FISH=fluorescence in situ hybridization.
|
Table 2
Demonstration of SISH vs FISH HER2 gene amplification status obtained from 58 consecutively examined invasive breast cancer cases

Table 3
Multiple comparison between HER2 amplification determined by SISH, FISH and HER2 overexpressed by IHC (TMA, Ventana)

References
1. Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986. 232:1644–1646.

2. Ross JS, Fletcher JA. HER-2/neu (c-erb-B2) gene and protein in breast cancer. Am J Clin Pathol. 1999. 112:S53–S67.
3. Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999. 17:2639–2648.

4. Shak S. Herceptin Multinational Investigator Study Group. Overview of the trastuzumab (Herceptin) anti-HER2 monoclonal antibody clinical program in HER2-overexpressing metastatic breast cancer. Semin Oncol. 1999. 26:71–77.
5. Press MF, Sauter G, Bernstein L, Villalobos IE, Mirlacher M, Zhou JY, et al. Diagnostic evaluation of HER-2 as a molecular target: an assessment of accuracy and reproducibility of laboratory testing in large, prospective, randomized clinical trials. Clin Cancer Res. 2005. 11:6598–6607.

6. Lewis F, Jackson P, Lane S, Coast G, Hanby AM. Testing for HER2 in breast cancer. Histopathology. 2004. 45:207–217.

7. Dietel M, Ellis IO, Höfler H, Kreipe H, Moch H, Dankof A, et al. Comparison of automated silver enhanced in situ hybridisation (SISH) and fluorescence ISH (FISH) for the validation of HER2 gene status in breast carcinoma according to the guidelines of the American Society of Clinical Oncology and the College of American Pathologists. Virchows Arch. 2007. 451:19–25.

8. Shousha S, Peston D, Amo-Takyi B, Morgan M, Jasani B. Evaluation of automated silver-enhanced in situ hybridization (SISH) for detection of HER2 gene amplification in breast carcinoma excision and core biopsy specimens. Histopathology. 2009. 54:248–253.

9. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007. 25:118–145.

10. Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989. 244:707–712.

11. Révillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998. 34:791–808.

12. Tandon AK, Clark GM, Chamness GC, Ullrich A, McGuire WL. HER-2/neu oncogene protein and prognosis in breast cancer. J Clin Oncol. 1989. 7:1120–1128.

13. Mass RD, Press MF, Anderson S, Cobleigh MA, Vogel CL, Dybdal N, et al. Evaluation of clinical outcomes according to HER2 detection by fluorescence in situ hybridization in women with metastatic breast cancer treated with trastuzumab. Clin Breast Cancer. 2005. 6:240–246.

14. Tubbs RR, Pettay JD, Roche PC, Stoler MH, Jenkins RB, Grogan TM. Discrepancies in clinical laboratory testing of eligibility for trastuzumab therapy: apparent immunohistochemical false-positives do not get the message. J Clin Oncol. 2001. 19:2714–2721.

15. Arnould L, Arveux P, Couturier J, Gelly-Marty M, Loustalot C, Ettore F, et al. Pathologic complete response to trastuzumab-based neoadjuvant therapy is related to the level of HER-2 amplification. Clin Cancer Res. 2007. 13:6404–6409.

16. Carbone A, Botti G, Gloghini A, Simone G, Truini M, Curcio MP, et al. Delineation of HER2 gene status in breast carcinoma by silver in situ hybridization is reproducible among laboratories and pathologists. J Mol Diagn. 2008. 10:527–536.

17. Itoh H, Kato N, Serizawa A, Itoh T, Umemura S, Osamura RT. HER2 rabbit monoclonal antibody 4B5 and silver SISH: high performance in surgical pathology for appropriate patient care. Mod Pathol. 2009. 22:205.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download