INTRODUCTION
Malignant peripheral nerve sheath tumors (MPNSTs) are a very rare neoplasm, and they represent only 5-10% of all the malignant soft tissue sarcomas. These tumors may arise sporadically in adult patients, but these tumors frequently occur in patients suffering with neurofibromatosis Type I (NF 1). The mean age at the time of diagnosis of MPNST is in the thirties, but the patients with NF 1 are about 10 yr younger than the patients without NF 1.(1) MPNSTs are also known to have an association with previous irradiation. MPNSTs are known to arise within the field of irradiation, 9 to 36 yr after radiation therapy administered for treating previous malignancies.(2) MPNSTs are commonly found in the trunk and the extremities (51% and 45% of the patients, respectively) and in the head and neck in 4% of patients.(3) Most primary tumors of the breast have an epithelial origin, and a MPNST arising in the breast is an extremely rare finding. In our review of the literature on MPNSTs, only 9 cases have been reported from 1983 to the present. We report here on our clinical experience of a patient who presented with a solitary MPNST of the breast, and we treated this tumor with surgical excision and radiation therapy.
CASE REPORT
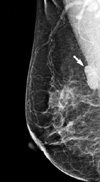
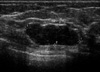
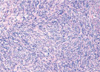
A 59-yr-old woman presented to our hospital complaining of a mass in the right breast. The mass was non-tender, it was firm-to-hard in consistency, approximately 3 cm in size and it was located at the 10 o'clock position in the right breast. She had no history of a prior breast mass, trauma, nipple discharge, mastalgia or a family history of breast cancer, and did not present any features of neurofibromatosis. Mammography demonstrated a well defined ovoid mass shadow that was just onto the pectoralis major muscle (Figure 1). Ultrasonography of the breast showed a macrolobulating hypoechoic mass in the upper outer quadrant of the right breast, and its size measured 2.8×1.0 cm (Figure 2). She was admitted for excisional biopsy and she underwent the operation under general endotracheal anesthesia. At the time of surgery, the tumor was found in the deep portion of the right breast, and it was firmly attached to the pectoralis major muscle. The mass was completely excised. The excised surgical specimen consisted of a well-circumscribed, firm, solid mass measuring 2.5×1.2×1.1 cm. Its cut surface was whitish gray, smooth and myxoid and it had prominent fibrous strands. No necrosis, hemorrhage or cystic degeneration was grossly identified. Histologically, the neoplasm consisted of spindle cells surrounded by a fibrous stroma (Figure 3). The mitotic count revealed 19 mitosis/10 high-power fields. Immunochemical staining was performed on the tissue section with S-100, vimentin, desmin, actin and cytokeratin. The immunohistochemical staining of S-100 protein was diffusely positive in the tumor cells (Figure 4). Smooth muscle actin, CD 34 and CD 68 were non-reactive in the tumor cells. So we could exclude the possibility of other spindle cell tumors including smooth muscle tumor, solitary fibrous tumor, vascular tumor and malignant fibrous histiocytoma. On the basis of the cytohistologic appearance and the immunohistochemical pattern, this tumor was interpreted to be a spindle cell neoplasm with neural differentiation, and this was suggestive of a MPNST. A wide excision around the previous operation site was performed and the patient postoperatively received radiation therapy to the right breast. She had no complication during the treatment, and she remains well without signs of local recurrences or distant metastases 1 yr following her surgery.
DISCUSSION
Peripheral nerve tumors are infrequently encountered soft tissue lesions that can affect any organ of the body and so they have a myriad of differential diagnoses. MPNST is classified into the primary malignant subtype of the peripheral nerve.(4) The incidence of MPNSTs is 0.001% in the general population, but 5% to 42% of the cases have an association with neurofibromatosis type 1 (NF 1). MPNSTs arise in adult patients who range in age from 20 to 50 yr of age. They originate from a major or minor peripheral nerve branch or its sheath. The most common sites of presentation of MPNSTs are the trunk, followed by the extremities and the head and neck.(1,3,5) MPNST of the breast as a primary tumor is very rare and has been only eight such reported cases in the scope of our search,(6-13) and two cases among them were associated with neurofibromatosis.(7,9)
There are no specific symptoms or signs other than a palpable lump in the breast, and making the correct preoperative diagnosis of MPNST tumor is difficult. The initial diagnoses by fine needle aspiration cytology in the cases of Catania et al.(6) and Dhingra et al.(9) were a mesenchymal tumor and a spindle cell tumor, respectively. The initial diagnosis by excisional biopsy in the case of Malas et al.(7) was fibrous histiocytoma. In the cases of Medina-Franco et al.,(8) the diagnosis of MPNST was made by excision and immunohistochemical staining. The clinical relevance of these cases shows the importance of harvesting enough tissue for histologic analysis and immunohistochemical staining. MPNST have to be distinguished from malignant phyllodes tumor, fibrosarcoma and leiomyosarcoma. We could rule out these other tumors by the immunohistochemical staining. The definite treatment for this tumor is only complete surgical resection and the prognosis of patients with MPNST is significantly determined by whether or not complete resection has been achieved.(1) The principles for the management of MPNSTs are similar to those for the management of all types of soft tissue sarcomas.(14) The goal of operation is complete removal of the tumor with histologically clear resection margins. Although some authors recommended a mastectomy for the primary therapy of MPNST of the breast,(6,7) the extent of surgery remains uncertain due to its rarity. But axillary dissection is not indicated as the regional lymph nodes are not usually affected.(7) There are no reports in the literature on the role of radiotherapy or chemotherapy for the treatment of MPNST of the breast. In the present case, we performed wide excision and radiotherapy as there was thought to be a risk of recurrence related to the highly mitotic features of the tumor. The prognosis for patients with MPNST remains poor. The reported 5-yr survival rates were 34-40% in two studies,(1,3) and the unfavorable features for recurrence are the tumor size, the site of origin, and the margin status.(3) MPNSTs associated with NF 1 are more aggressive and they have a worse prognosis following local recurrence than do the tumors without NF 1.(15)
In conclusion, MPNST of the breast is often unsuspected and the diagnosis may be missed unless clinicians have awareness of this disease. Clinicians should pay special attention when a patient with the stigmata of neurofibromatosis type 1 (i.e., multiple cutaneous nodules, café au lait spots, freckling in the axilla or groin) presents with a mass in the breast, clinicians should use special attention for it. Although we performed wide excision and radiotherapy in this case, the optimum treatment is still not clear as the experience with this rare tumor is limited.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download