Abstract
An adenomyoepithelioma (AME) is an uncommon neoplasm characterized by proliferation of both epithelial and myoepithelial cells in the salivary gland, skin, lung and breast. AMEs can recur, progress to malignancy and metastasize. A 68-year-old woman presented a large mass occupying her whole right breast. The mass had grown slowly for about 20 years and the preoperative biopsy of the mass was chondroid syringoma. The mass was completely resected and the postoperative biopsy revealed malignant AME with a negative resection margin. The patient didn't receive any adjuvant therapy and has been free of recurrence or metastasis up to now. We report herein a case of a malignant AME that was diagnosed in the largest breast mass reported to date. This mass grew slowly and without metastasis. Clinicians should consider this rare disease entity in the differential diagnosis of a breast mass and remember the importance of complete excision of this tumor.
An adenomyoepithelioma (AME) is characterized by a biphasic proliferation of both epithelial and myoepithelial cells. AME has been reported to occur in breast, salivary gland, lung and skin.(1) An AME of the breast is a rare tumor and there are less than 200 such reported cases.(2,3) Malignancy arising from AME of the breast is much rarer and only about 20 such cases have been reported in the literature.(3,4) Metastasis of an AME is unusual, but this has been reported even in benign cases.(5) The reported sizes of these tumors are most less than 5 cm. In this report, we describe a huge breast mass that was diagnosed as a malignant AME and was completely excised.
A 68-yr-old woman was transferred to our clinic, for the treatment of a large mass in the right breast. She had no history of breast disease other than the mass and no history of medical diseases except hypertension. The mass had grown slowly for about 20 yr, but the patient had not sought evaluation. As the mass grew, the center of the mass became ulcerated, leading to necrosis and a skin defect, but she had continued to dress the wound herself. She visited another hospital 1 week before she was referred to our hospital. A biopsy of the mass at the other hospital revealed a chondroid syringoma. The attending physician transferred her to our clinic in the hope that she could undergo a flap procedure after resection of the mass.
The mass was 15 cm at the maximal diameter and it occupied the entire right breast (Figure 1A). The nipple and areola were absent as a result of the necrosis. The area of necrosis was approximately 8 cm at the maximal diameter. The mass was not fixed to the chest wall. No axillary lymph nodes were palpated. Neither mammography nor breast ultrasound was performed. The mass had an opaque lesion on the chest X-ray. The pathologist at our hospital reviewed the slide of the biopsy specimen and made the diagnosis of myoepithelioma: no malignant component was not identified on the slide.
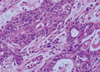
We performed a total mastectomy of the right breast. The skin was closed by primary repair after undermining without a flap procedure (Figure 1B). Grossly, the tumor was a multinodular solid mass with focal cystic change. The cut surface was pale yellow, soft to fish-flesh and partly myxoid. It involved the overlying skin, and this resulted in skin ulceration (Figure 2). Microscopic examination showed a proliferation of epithelial cells invested by myoepithelial cells, forming a tubular or trabecular growth pattern (Figure 3). Multiple foci of the tumor showed cytologic atypia, increased mitotic activity and overgrowth of the glandular component (Figure 4). The final pathologic diagnosis was malignant AME. A negative resection margin was confirmed by the pathologic examination.
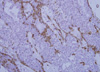
Immunohistochemical study demonstrated that the myoepithelial cells were reactive for smooth muscle actin (Figure 5), p63 and cytokeratin 5/6, partly reactive for S-100 protein and negative for cytokeratin 7 and carcinoembryonic antigen, whereas the epithelial cells were positive for cytokeratin 7 and negative for myoepithelial markers. The Ki-67 index was increased up to 10%. The tumor cells were intermediately positive for estrogen receptor and negative for progesterone receptor and c-erb-B2.
The patient recovered without any wound complications. A positron emission tomography-computed tomography (PET-CT) scan was done after discharge and showed no evidence of remnant tumor or metastasis. She declined chemotherapy and hormone therapy. The radiation oncologist recommended radiation therapy, but she did not want it. She has been free of recurrence for 5 months after surgery.
Hamperl(6) first described an AME of the breast in 1970. Tavassoli(7) classified benign AMEs into the spindle cell, tubular and lobulated types in 1991. Making the preoperative diagnosis by fine needle aspiration cytology or core biopsy is difficult due to the presence of a dual cell population.(8) A chondroid syringoma is a benign mixed tumor that has a hyalinized or chondromyxoid stroma and can be misdiagnosed on the preoperative biopsy of an AME, as happened in this report.(9) The differential diagnosis also includes adenoid cystic carcinomas, spiradenomas, tubular apocrine adenomas, fibroadenomas and even phyllodes tumors.(8-10) The rarity of this disease adds to the possibility of preoperatively misdiagnosing this tumor on the core needle biopsy or on the fine needle aspiration cytology. Immunohistochemical examination that includes smooth muscle actin, p63, desmin, vimentin and S-100 protein aids in making the definitive diagnosis of AMEs.(11)
Most AMEs are characterized by a well-circumscribed solid tumor with a fibrous pseudocapsule and multinodularity, although a case of intracystic AME of the breast has been reported.(3) AMEs of the breast usually present as a solitary mass, but multiple foci of AMEs with various subtypes have also been reported.(12) The mammographic, sonographic and MRI findings of AMEs are neither specific nor helpful for making the preoperative diagnosis, or to differentiate between benign and malignant lesions.(13)
Most AMEs are benign. The diagnosis of malignant AMEs has been applied to tumors with cytologic atypia, local invasion and high mitotic activity, regardless of metastasis.(4) Malignant transformation may arise from the epithelial component (as in this current case), the myoepithelial component or both components.(4) The World Health Organization has classified AMEs into epithelial carcinomas, myoepithelial carcinomas, biphasic epithelial and myoepithelial carcinomas, sarcoma and carcinosarcomas.(14)
An AME of the breast is usually manifested as a painless mass. The sizes of the breast mass have ranged from 0.5 cm to 7 cm.(2) The mass can show a tendency toward rapid enlargement, unlike the case reported herein. Our patient presented with the largest size of this kind of tumor that has been ever reported. In this case, the tumor was supposed to be transformed to malignancy during the growth. The reported age range at the time of diagnosis is 26-80 yr. An AME in the male breast is extremely rare and only three benign cases have been reported.(13)
AMEs rarely metastasize and they have a tendency to spread hematogeneously. The reported sites of metastasis include the axillary lymph nodes, lung, brain, bone and thyroid.(5,7,12,13,15) Lung metastases have been reported in 2 cases of benign AMEs.(5) One case of thyroid metastasis was detected 12 yr after excision of the breast lesion.(15) Mortality has occurred within a few years in the reported cases with metastasis of malignant AMEs.(4)
There is no established treatment for AMEs except surgical removal. Local recurrence and distant metastasis after complete excision have been reported.(5) Chemotherapy has been tried, but it is not effective.(12) The effects of hormone therapy and radiotherapy are not proven. In the case of distant metastasis, local recurrence from incomplete excision is more common, and this contributes to a poor prognosis.(4) The overall prognosis of AME is not known due to the rarity of the disease.
An AME is a rare breast tumor with various clinical manifestations. We report here on a case of a malignant AME that presented as a large tumor of the breast without distant metastasis; the tumor was completely excised and we misdiagnosed it preoperatively. Clinicians need to be aware of AMEs as part of the differential diagnosis of breast tumors, and they must aware of the importance of complete excision because of the tumor's potential for recurrence and metastasis. Although surgery is the only proven treatment modality, treatment that includes chemotherapy, hormone therapy and radiation therapy may contribute to improving the prognosis of this disease entity.
Figures and Tables
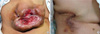 | Figure 1The preoperative (A) and postoperative (B) appearance of the right breast. (A) The necrotized area of the central portion was broad, and it included the nipple and areola. (B) The operative wound was clean without complications after complete excision of the mass and primary repair. |
 | Figure 2Cut surface of the tumor. It showed multinodularity with fibrous septae and skin invasion that caused ulceration. |
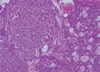 | Figure 3Microscopic finding of the tumor. The tumor showed vaguely nodular growth of proliferating epithelial cells invested by myoepithelial components forming tubules or trabeculae (H&E stain, ×40). |
References
2. Yahara T, Yamaguchi R, Yokoyama G, Yamaguchi M, Nakagawa S, Toh U, et al. Adenomyoepithelioma of the breast diagnosed by a mammotome biopsy: report of a case. Surg Today. 2008. 38:144–146.

3. Hikino H, Kodama K, Yasui K, Ozaki N, Nagaoka S, Miura H. Intracystic adenomyoepithelioma of the breast: case report and review. Breast Cancer. 2007. 14:429–433.

4. Ahmed AA, Heller DS. Malignant adenomyoepithelioma of the breast with malignant proliferation of epithelial and myoepithelial elements: a case report and review of the literature. Arch Pathol Lab Med. 2000. 124:632–636.

5. Nadelman CM, Leslie KO, Fishbein MC. "Benign," metastasizing adenomyoepithelioma of the breast: a report of 2 cases. Arch Pathol Lab Med. 2006. 130:1349–1353.

6. Hamperl H. The myothelia (myoepithelial cells). Normal state; regressive changes; hyperplasia; tumors. Curr Top Pathol. 1970. 53:161–220.
7. Tavassoli FA. Myoepithelial lesions of the breast: myoepitheliosis, adenomyoepithelioma and myoepithelial carcinoma. Am J Surg Pathol. 1991. 15:554–568.
8. Iyengar P, Ali SZ, Brogi E. Fine-needle aspiration cytology of mammary adenomyoepithelioma: a study of 12 patients. Cancer. 2006. 108:250–256.

10. Chang A, Bassett L, Bose S. Adenomyoepithelioma of the breast: a cytologic dilemma. Report of a case and review of the literature. Diagn Cytopathol. 2002. 26:191–196.

11. Schürch W, Potvin C, Seemayer TA. Malignant myoepithelioma (myoepithelial carcinoma) of the breast: an ultrastructural and immunocytochemical study. Ultrastruct Pathol. 1985. 8:1–11.

12. Chen PC, Chen CK, Nicastri AD, Wait RB. Myoepithelial carcinoma of the breast with distant metastasis and accompanied by adenomyoepitheliomas. Histopathology. 1994. 24:543–548.

13. Park MH. Malignant adenomyoepithelioma of the breast. J Korean Surg Soc. 2007. 73:430–433.
14. Tavassoli FA, Devilee P. World Health Organization classification of tumours: pathology and genetics of tumours of the breast and female genital organs. 2003. Lyon: IARC Press.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download