Abstract
Purpose
Breast cancer has been reported as the most common cancer of women in the United States, Western Europe and Korea and about 210,000 and 10,000 women in United States and Korea every year, respectively are diagnosed with it. Breast cancer is curable with an early diagnosis, and many researchers have made efforts to find a marker for this malady, heat shock protein (HSP) consists of 6 groups, it is highly preserved throughout both the prokaryotic and eukaryotic cells and it acts as a molecular chaperone that's involved in protein folding. HSPs have been recently reported to be related with breast cancer. In this study, we investigated the changes of expression of HSP60 in tissues and cell lines of breast cancer.
Methods
We obtained breast cancer tissues and normal tissues from breast cancer patients, and we purchased several cancer cell lines from American tissue culture correction. We treated the tissues and the cell lines of human breast cancer with heat shock protein. Proteins and mRNAs were isolated from the tissues and the cells and then we performed Western blotting, reverse transcriptase-Polymerase chain Reaction and fluorescence activated cell sorter analysis on them.
Results
On Western blot, HSP60 was more overexpressed in the tissues and the cell lines of breast cancer than in the normal breast tissues and cell lines. The expression of HSP60 showed 2 types of molecular weight differences in the tissues and cell lines of breast cancer, and specifically, low HSP60 was over-expressed in the cancer tissues. There was no difference between the breast cancer cell lines and the normal cell lines in the expressions of HSP60 mRNA, according to the treatment with heat shock. Also, there was no relationship between phosphorylation and the structural difference of HSP60 protein, according to HSP60 protein's molecular weight. The expression of HSP60 has been mostly reported at the mitochondria; however, in this study, it was more predominantly detected at the cytoplasm than at the mitochondria in the breast cancer cell lines.
Figures and Tables
 | Fig 1Structure of heat shock protein 60 kDa (HSP60). Molecular weight is 60 kDa, synthesized in the cytoplasm and transported to mitochondria. |
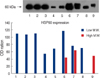 | Fig 2Expression of HSP60 in cell line and tissue by western blot. Lane 1=cancer cell line (37℃); lane 2=cancer cell line (41℃ heat shock treatment); lane 3=cancer cell line (45℃ heat shock treatment); lane 4=normal cell line (37℃); lane 5=normal cell line (41℃); lane 6=breast cancer tissue; lane 7=breast normal tissue; lane 8=cervix cancer tissue; lane 9=cervix normal tissue. |
 | Fig 3Expression of HSP60 in MDA-MB-231 by western blot. HSP60 was detected by western blotting using heat shock 60 kDa protein 1 (chaperonin) polyclonal antibody. (A) Expression pattern of HSP60 in MDA-MB-231 cell line treated heat shock (37, 39, 41, 43, 45℃). (B) Expression pattern of β-actin in MDA-MB-231 cell line treated heat shock (37, 39, 41, 43, 45℃).
BC=Breast Cancer tissue; BN=Breast Normal tissue.
|
 | Fig 4Expression of HSP60 in MCF-7 by western blot. HSP60 was detected by western blotting using heat shock 60 kDa protein 1 (chaperonin) polyclonal antibody. (A) Expression pattern of HSP60 in MCF-7 cell line treated heat shock (37, 39, 41, 43, 45℃). (B) Expression pattern of β-actin in MCF-7 cell line treated heat shock (37, 39, 41, 43, 45℃),
BC=Breast Cancer tissue; BN=Breast Normal tissue.
|
 | Fig 5Expression of HSP60 in CCD-1113 by western blot. HSP60 was detected by western blotting using heat shock 60 kDa protein 1 (chaperonin) polyclonal antibody. (A) Expression pattern of HSP60 in CCD-1113sk cell line treated heat shock (37, 39, 41, 43, 45℃). (B) Expression pattern of β-actin in CCD-1113sk cell line treated heat shock (37, 39, 41, 43, 45℃).
BC=Breast Cancer tissue; BN=Breast Normal tissue.
|
 | Fig 6Amplification of HSP60 and GAPDH in MDA-MB-231 by RT-PCR. A 2.5% agarose gel electrophoresis of RT-PCR products of MDA-MB-231 cell line mRNA. RT-PCR was carried out using the primer pair HSP60 and GAPDH. Lane M.W, size marker (phix174/BsuRI [HaeIII]). (A) RT-PCR products of HSP60 in MDA-MB-231 cell line treated with heat shock (37, 39, 41, 43, 45℃). (B) RT-PCR products of GAPDH in MDA-MB-231 cell line treated with heat shock (37, 39, 41, 43, 45℃).
BC=Breast Cancer tissue; BN=Breast Normal tissue.
|
 | Fig 7Amplification of HSP60 and GAPDH in MCF-7 cell line by RT-PCR. A 2.5% agarose gel electrophoresis of RT-PCR products of MCF-7 cell line mRNA. RT-PCR was carried out using the primer pair HSP60 and GAPDH. Lane M.W, size marker (phix174/BsuRI [HaeIII]). (A) RT-PCR products of HSP60 in MCF-7 cell line treated with heat shock (37, 39, 41, 43, 45℃). (B) RT-PCR products of GAPDH in MCF-7 cell line treated with heat shock (37, 39, 41, 43, 45℃).
BC=Breast Cancer tissue; BN=Breast Normal tissue.
|
 | Fig 8Amplification of HSP60 and GAPDH in CCD-1113 by RT-PCR. A 2.5% agarose gel electrophoresis of RT-PCR products of CCD-1113sk cell line mRNA. RT-PCR was carried out using the primer pair HSP60 and GAPDH. Lane M.W, size marker (phix174/BsuRI [HaeIII]). (A) RT-PCR products of HSP60 in CCD-1113sk cell line treated heat shock (37, 39, 41, 43, 45℃). (B) RT-PCR products of GAPDH in CCD-1113sk cell line treated heat shock (37, 39, 41, 43, 45℃).
BC=Breast Cancer tissue; BN=Breast Normal tissue.
|
 | Fig 9Treatment of alkaline phosphatase (AP). (A) Expression of HSP60 in MDA-MB-231 cell line treated heat shock (37, 39, 41, 43, 45℃) by western blot. (B) Before alkaline phosphatase treatment. Lane 1=cancer cell line (37℃); lane 2=cancer cell line (41℃ heat shock treatment); lane 3=cancer cell line (45℃ heat shock treatment); lane 4=normal cell line (37℃); lane 5=normal cell line (41℃); lane 6=breast cancer tissue; lane 7=breast normal tissue. (C) After Alkaline Phosphatase treatement. Lane 1=cancer cell line (37℃); lane 2=cancer cell line (41℃ heat shock treatment); lane 3; cancer cell line (45℃ heat shock treatment); lane 4=normal cell line (37℃); lane 5=normal cell line (41℃); lane 6=breast cancer tissue; lane 7=breast normal tissue. |
 | Fig 10Extraction of cell membrane, nucleus and mitochondria. Lane 1=Nucleus protein extracted in cancer cell line; lane 2=Mitochondria protein extracted in cancer cell line; lane 3=cancer cell line; lane 4=normal cell line; lane 5=breast cancer tissue; lane 6=breast normal tissue. |
 | Fig 11Cytofluorimetric analysis of Mitofluor in living cell. FACS can analysis was performed with an direct method. Samples were acquired using a flow cytometer and data were analyzed with the Lysis II software (FACScan, Becton-Dickinson). (A) Normal, (B) Cancer, (C) compare cancer with normal cell line. |
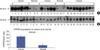 | Fig 12Expression of HSP60 and β-actin in Breast cancer and normal tissue by western blot. HSP60 was detected by western blotting using heat shock 60 kDa protein 1 (chaperonin) polyclonal antibody. (A) Expression pattern of HSP60 in breast cancer (1-6, 10-14, 18-26) and normal tissues (7-9, 15-17). (B) Expression pattern of β-actin in breast cancer (1-6, 10-14, 18-26) and normal tissues (7-9, 15-17). |
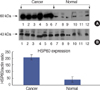 | Fig 13Expression ratio of HSP60 and β-actin in Breast cancer and normal tissue by western blot. HSP60 was detected by western blotting using heat shock 60 kDa protein 1 (chaperonin) polyclonal antibody and β-actin antibody. (A) Expression pattern of HSP60 in breast normal and cancer tissues and (B) is β-actin (ANOVA t test [Mann Whitney], p<0.0001). |
References
2. Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtiqian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994. 266:66–71.

4. Ritossa FA. A new puffing pattern induced by a temperature shock and DNP in Drosophila. Experientia. 1962. 18:571–573.

5. Tissiere A, Mitchell HK, Tracy U. Protein synthesis in salivary glands of Dorsophila melanogaster: relation to chromosomal puffs. J Mol Biol. 1974. 84:389–398.
7. Cappello F, Bellafiore M, Palma A, Marciano V. Expression of 60-kD heat shock protein increases during carcinogenesis in the uterine exocervix. Pathobiology. 2002. 70:83–88.

9. Morano KA, Thiele DJ. Heat shock function and regulation in response to cellular stress, growth, and differentiation signals. Gene Expr. 1999. 7:271–282.
10. Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000. 92:1564–1572.

11. Knowlton AA. The role of heat shock proteins in the heart. J Mol Cell Cardiol. 1995. 27:121–131.

12. Schneider J, Jimenez E, Marenbach K, Romero H, Mark D, Meden H. Immunohistochemical detection of HSP60-expression in human ovarian cancer, correlation with survival in a series of 247 patients. Anticancer Res. 1999. 19:2141–2146.
13. Srivastava PK, Maki RG. Stress-induced proteins in immune response to cancer. Curr Top Microbiol Immunol. 1991. 167:109–123.

14. Bradford MM. A rapid and sensitive methods for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binging. Anal Biochem. 1976. 72:248–254.

15. Itoh H, Kobayashi R, Wakui H, Komatsuda A, Ohtani H, Miura AB, et al. Mammalian 60-kDa stress protein (chaperonin homolog). identification, biochemical properties, and localization. J Biol Chem. 1995. 270:13429–13435.
16. Franzen B, Linder S, Alaiya AA, Eriksson E, Fujioka K, Berman AC, et al. Analysis of polypeptide expression in benign and malignant human breast lesions. Electrophoresis. 1997. 18:582–587.

17. Morimoto RI. Heat shock: the role of transient inducible responses in cell damage, transformation, and differentiation. Cancer Cells. 1991. 3:295–301.
18. Itoh H, Komatsuda A, Ohtani H, Wakui H, Imai H, Sawada K, et al. Mammalian HSP60 is quickly sorted into the mitochondria under conditions of dehydration. Eur J Biochem. 2002. 269:5931–5938.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download