Abstract
Ovarian carcinoma usually metastasizes to the peritoneal cavity and the regional lymph nodes. While the peritoneal metastases if often widespread, metastases beyond the peritoneal cavity including axillary lymph nodes are uncommon. A recurrence of ovarian carcinoma in only the axillary lymph node is also a rare case. We report here on an axillary lymphatic metastasis as a result of recurrence of ovarian carcinoma in a 43-yr-old woman. Six years after the initial operation for ovarian carcinoma, multiple lymphatic metastases in the right axilla were noted as a palpable axillary masses. There are few previous reports about ovarian carcinoma that metastatized to the axillary lymph nodes. It must be differentiated from breast carcinoma because the treatment and prognosis of metastatic ovarian carcinoma differ from those of primary breast carcinoma.
Ovarian cancer is the fifth most common gynecologic carcinoma and one of the main causes of death from gynecologic malignancies.(1) Ovarian cancer generally presents with already advanced disease at the time of diagnosis and is known to have a poor prognosis due to such lack of symptom. A recurrence of ovarian cancer is commonly seen in the abdominopelvic cavity. The majority of described cases are associated with widespread malignancy and usually discovered incidentally at autopsy.(2, 3) There are primary breast and ovrian cancer commonly coexisting synchronously or metachronously, but metastases of ovarian carcinoma to axillary lymph node and breast are rare.(4-6) Since the incidence of metastatic ovarian cancer to the axillary lymph node is low, it is of great difficult to differentiate the metastatic cancer from primary breast cancer. However, its recognition and distinction from breast carcinoma are important because the treatment and prognosis of metastatic ovarian carcinoma differ from primary breast carcinoma significantly. Therefore, we report an experience of ovarian cancer patient with recurrence in axillary lymph node only.
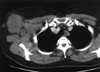
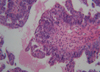
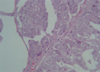
In April 2006, a 43-yr-old woman visited for a recently rapidly growing tender mass in her anterior axillary area. Physical examination showed a fixed hard mass, 8 cm in diameter, on the upper anterior axillary line. There was no papable mass on physical examination of breast, except for multiple enlarged lymph nodes. The mass has been palpated since January 2005, and started to grow aggressively since 3 months ago. Fine-needle aspiration cytology of the mass was undertaken, and cytology assessment revealed no malignant cell but reaspiration or biopsy was recommended due to inadequate specimen. Two view of mammography and ultrasonogram showed no abnormal finding in both breasts but multiple enlarged lymph nodes were noted on right axilla. Chest computed tomography scan also demonstrated multiple lymph node enlargement on right axillary area (Fig 1). As both breasts were normal on mammography and physical examination, an intensive diagnostic work up was performed to find a primary tumor. The patient had a past medical history of a pelvic mass in March 2000, when she underwent laparotomy, total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, optimal tumor debulking, and lymphadenectomy. The mass turned out to be poorly differentiated papillary serous ovarian adenocarcinoma in stage lllC (Fig 2). She subsequently had taken cycles of paclitaxel and carboplatin. In December 2003, her CA-125 serum level was elevated to 123 U/mL. Although metastatic work-up, including abdomino-pelvic computed tomography and bone scan, was undertaken, no recurrence or metastasis was found. She began combination chemotherapy comprised of six cycles of paclitaxel, carboplatin and gemcitabin. Her CA 125 declined to 32 U/mL in March 2004. We performed biopsy of axillary lymph node, which revealed metastatic adenocarcinoma. Histologic comparison of the ovarian tumor with the present axillary lymph nodes found them to be identical (Fig 3). Axillary dissection was done and fourteen lymph nodes were removed and found to have metastatic disease. The results of imunohistochemistry, ER, PR, Bcl-2, p53, c-Erb-B2 were all negative. After the operation, the patient's CA 125 declined from 437 to 91.7 U/mL. CT of the abdomen/pelvis and bone scan were both negative for additional metastasis to other organs.
Ovarian carcinoma spread primarily by seeding of cells into the peritoneal cavity, by lymphatic dissemination and by hematogenous spread. Hematogenous dissemination at the time of diagnosis is less commonly seen. The major involved organs reported were liver, lung, and pleura.(7, 8) Remained minor sites include skin, spleen, and central nervous system.(7, 9, 10) Most of the patients in whom metastatic disease develops have a known history of advanced stage ovarian or peritoneal carcinoma. The major cause of axillary metastatic lymphadenopathy presenting as adenocarcinoma in woman is the breast carcinoma.(11) The histologic features of breast and ovarian carcinoma may be quite similar and therefore great difficulty arises in making the differential diagnosis. However, clinical features, presence of ductal component and especially comparison of the previous slides may help to distinguish breast carcinoma from metastatic ovarian carcinoma. In our case, histologic comparison of axillary tumor and the previous ovarian carcinoma found them to be identical. The use of immunohistochemical markers such as cytokeratins, hormonal receptors, WT-1, GGCDFP-15 or other substances may assist in determining the origin of the tumor. (12-14) Estrogen receptor, progesterone receptor, and C-erb B2 were all negative in our case. In a case of isolated axillary nodal metastatic disease and in peritoneal papillary adenocarcinomatosis, serum Ca 15-3 and CA 125 could be of some help.(15) This patient's CA 125 was checked at 437 U/mL preoperatively, which strongly suggested a recurrence of ovarian carcinoma. Euscher et al. (4) examined 35 cases of serous carcinoma of the ovary, fallopian tube, or peritoneum presenting as lymphadenopathy and reported that extra-abdominal lymphadenopathy is not an adverse prognostic indicator and that the absence of bulky peritoneal disease appears to be a positive predictor of improved survival. But others reported that the breast and/or axillary lymph node metastases were discovered on an average of 30 months after presentation and the survival of theses cases was 13 months in average.(6) Surgical therapy for metastatic disease may be limited in the diagnostic biopsy to accurate differential diagnosis, because systemic chemotherapy is the choice of therapy. However, local surgery is another option of a palliative measure for patients who are resistant to chemotherapy or those who refuse chemotherapy. In conclusion, when a patient presents with metastatic disease, it is important to determine the origin of the metastatic tumor, since chemotherapeutic regimen for these carcinomas, breast and ovarian, are different. Although ovarian carcinoma metastasized to axillary lymph node is rare, we draw attention to the suspicion of metastatic ovarian carcinoma.
Figures and Tables
References
1. Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics, 2001. CA Cancer J Clin. 2001. 51:15–36.

4. Euscher ED, Silva EG, Deavers MT, Elishaev E, Gershenson DM, Malpica A. Serous carcinoma of the ovary, fallopian tube, or peritoneum presenting as lymphadenopathy. Am J Surg Pathol. 2004. 28:1217–1223.

5. Hockstein S, Keh P, Lurain JR, Fishman DA. Ovarian carcinoma initially presenting as metastatic axillary lymphadenopathy. Gynecol Oncol. 1997. 65:543–547.

6. Recine MA, Deavers MT, Middleton LP, Silva EG, Malpica A. Serous carcinoma of the ovary and peritoneum with metastases to the breast and axillary lymph nodes: a potential pitfall. Am J Surg Pathol. 2004. 28:1646–1651.

7. Bonnefoi H, A'Hern RP, Fisher C, Macfarlane V, Barton D, Blake P, et al. Natural history of stage IV epithelial ovarian cancer. J Clin Oncol. 1999. 17:767–775.

8. Cormio G, Rossi C, Cazzolla A, Resta L, Loverro G, Greco P, et al. Distant metastases in ovarian carcinoma. Int J Gynecol Cancer. 2003. 13:125–129.

9. Ro DY, Kim YW, Kim TE, Lee HY, Chung DS, Lee A. A case of cerellar metastases from ovarian carcinoma. Korean J Obstet Gynecol. 2003. 46:153–157.
10. Gemignani ML, Chi DS, Gurin CC, Curtin JP, Barakat RR. Splenectomy in recurrent epithelial ovarian cancer. Gynecol Oncol. 1999. 72:407–410.

11. de Andrade JM, Marana HR, Sarmento Filho JM, Murta EF, Velludo MA, Bighetti S. Differential diagnosis of axillary masses. Tumori. 1996. 82:596–599.

12. Tot T. Cytokeratins 20 and 7 as biomarkers: usefulness in discriminating primary from metastatic adenocarcinoma. Eur J Cancer. 2002. 38:758–763.
13. Al-Hussaini M, Stockman A, Foster H, McCluggage WG. WT-1 assists in distinguishing ovarian from uterine serous carcinoma and in distinguishing between serous and endometrioid ovarian carcinoma. Histopathology. 2004. 44:109–115.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download