Abstract
Purpose
Infertility due to ovarian failure that is caused by antineoplastic chemotherapeutic agents is one of the primary problems of female cancer patients who are in their reproductive years. It has become important to preserve the reproductive potential of female cancer patients. This study was conducted to determine whether autotransplantation of frozen ovaries can restore reproductive potential.
Methods
This study included 30 female mice that had normal reproductive potential. The mice were divided into 4 groups: the positive control, the negative control, the comparison group, and the experimental group. The positive control group received right total oophorectomy, and the negative control group received bilateral total oophorectomy. Greater than or equal to 90% of the left ovary was removed in the mice of the comparison group, and then cyclophosphamide was administered. In the experimental group, the right ovary taken out by right total oophorectomy, and this was crypreserved using the vitrification method. And then cyclophosphamide was administered. The cryopreserved ovary was autotransplanted to the left gonadal fat pad after greater than or equal to 90% of the left ovary was removed. The reproductive performance in each group was analyzed according to the pregnancy rate after mating.
Results
In the positive control group, all five mice became pregnant, and the number of fetuses was 4 to 5 (mean= 4.60±0.55). In the comparison group, the pregnancy rate was 50%, and the mean number of fetuses was 1.40±0.55. In the experimental group, 7 of 10 (70%) mice became pregnant, and the mean number of fetuses was 4.71±2.56. There was no significant difference in the number of fetuses between the positive control and the experimental group (p=0.093), but there was a significant difference in the number of fetuses between the comparison group and the experimental group (p=0.019).
REFERENCES
1. Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at -196℃. Hum Reprod. 1994; 9:597–603.
2. Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. A Live birth after orthotopic transplatation of cryopreserved ovarian tissue. Lancet. 2004; 364:1405–1410.
3. Gunasena KT, Villines PM, Critser ES, Critser JK. Live births after autologous transplant of cryopreserved mouse ovaries. Hum Reprod. 1997; 12:101–106.

4. Meirow D, Lewin A, Or R, Rachmilewitz E, Slavin S, Schenker JG, et al. Ovarian failure post-chemotherapy in young cancer patients-risk assessment indicate the need for intervention. Fert Steril. 1997; 68 Suppl 1:S218.
5. Familiari G, Caggiati A, Nottola SA, Ermini M, Di Benedetto MR, Motta PM. Infertility: ultrastructure of human ovarian primordial follicles after combination chemotherapy for Hodgkin\'s disease. Hum Reprod. 1993; 8:2080–2087.

6. Trounson A, Mohr L. Human pregnancy following cryopreservation. thawing and transfer of an eight cell embryo. Nature. 1983; 305:707–709.
8. Ataya K, Ramahi-Ataya A. Reproductive performance of female rats treated with cyclophosphamide and/or LHRH agonist. Reprod Toxicol. 1993; 7:229–235.

9. Bramley TA, Menzies GS. Measurement of luteal and placental gonadotrophin-releasing hormone (GnRH) binding sites: role of inactivation of GnRH tracer. Mol Hum Reprod. 1996; 2:535–539.
10. Park MH, Yoon HC, Yoon JH, Jegal YJ. The efficacy of oral combination chemotherapy of 5\'-DFUR and cyclophosphamide for metastatic breast cancer. J Korean Breast Cancer Society. 2006; 9:249–253.

11. Nakao K, Nakagata N, Katsuki M. Simple and efficient vitrification procedure for cryopreservation of mouse embryo. Exp Anim. 1997; 46:231–234.
12. Parrot DM. The fertility of mice with orthotopic ovarian grafts derived from frozen tissue. J Reprod Fertil. 1960; 1:230–241.
13. Lee DM, Yeoman RR, Battaglia DE, Stouffer RL, Zelinski-Wooten MB, Fanton JW, et al. Live birth after ovarian tissue transplant. Nature. 2004; 428:137–138.

14. Oktay K, Karlikaya G, Gosden R, Schwarz R. Ovarian function after autologous transplantation of frozen-banked human ovarian tissue (abstract O-054). Fertil Steril. 1999; 72(3 Suppl 1):S21.
Fig 1.
Gross findings in the auto-transplanted ovary in the experimental group. (A) There are several fetuses in the left uterus after successful autotransplantation of cryopreserved ovary, (B) Even after successful autotransplantation of the cryopreserved ovary, no fetus is in the left uterus, (C) After autotransplantation of the cryopreserved ovary, there is no feus in the left uterus due to atrophic change of the autotransplanted ovary.
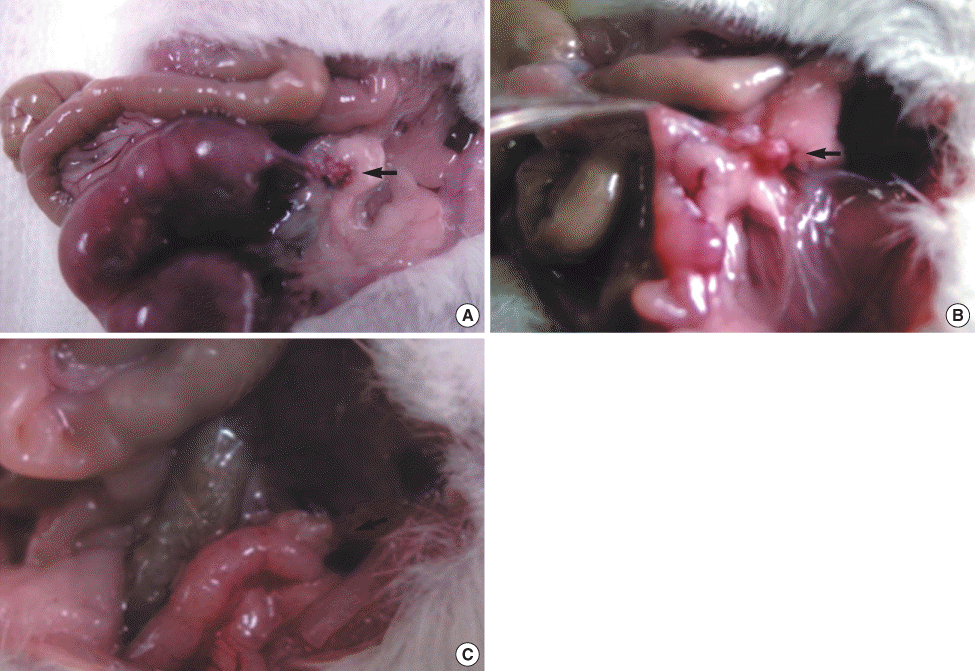
Fig 2.
A primary follicle in the the positive control group (A) and the experimental group (B) after transplantation (H&E stain, original magnification ×200). A primary follicle of the experimental group is alive and there is no significant difference in the structural components between the two groups.
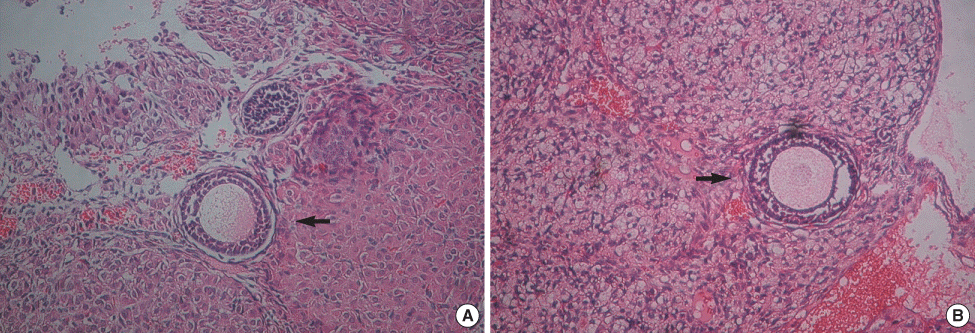
Fig 3.
H&E-stained ovaries. (A) An ovary in the positive control group has about 13 living oocytes, (B) an autografted ovary (right ovary) in the experimental group has about 5 living oocytes, (C) an atrophied ovary treated with cychlophosphamide (left ovary) in the experimental group has no oocytes (original magnification ×40).
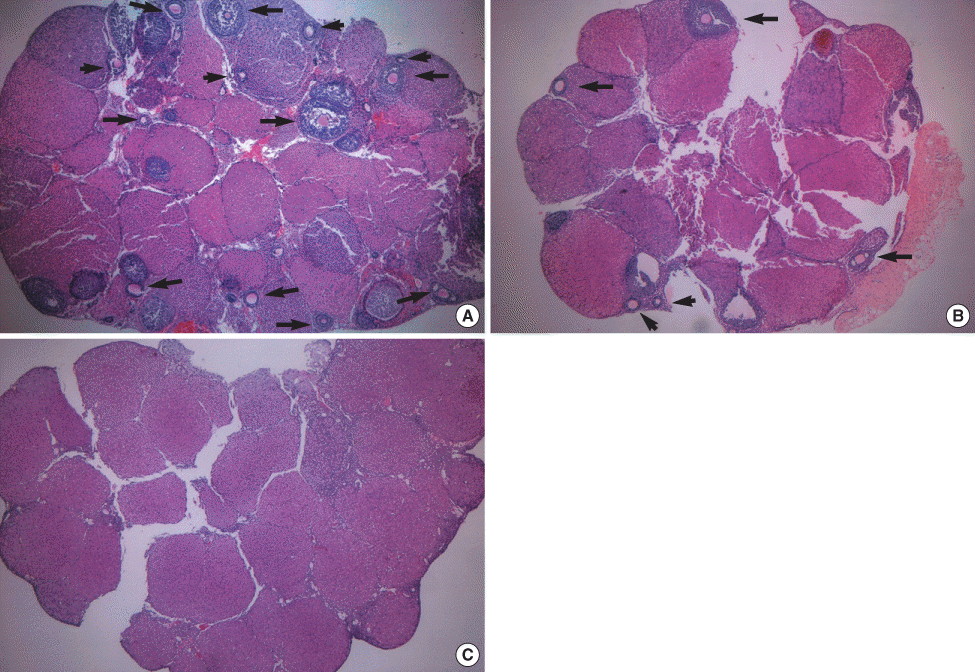
Table 1.
Time-frame of the experiment
| D1 | D3 | D4 | D5 | D7 | D15 | D21 | D28 | D37 | |
|---|---|---|---|---|---|---|---|---|---|
| Positive | RT.OX | Sham | Mating | ||||||
| Negative | BO.OX | Mating | |||||||
| Comparison | NT.OX | CY 250 | CY 250 | CY 250 | Mating | ||||
| Experimental | RT.OX, CP | CY 250 | CY 250 | CY 250 | LT.OX, AT | Mating |
D=postoperative day; RT.OX=Right total ovariectomy; BO.OX=Both total ovariectomy; NT.OX=Right total and left near-total ovariectomy; CP=Cryopreservation of resected right ovary; CY250=Intraperitoneal injection of cychlophosphamide 250 mg/kg; Sham=sham operation; LT.OX=Left neartotal ovariectomy; AT=Autotransplantation of cryopreserved ovary.
Table 2.
The number of the offsprings of successfully pregnant mice
Table 3.
Number of pups of successfully pregnant mice
Table 4.
The difference in the number of pups of successfully pregnant mice between the comparison group and the experimental group comparison group




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download