Abstract
Purpose
The Mammotome® biopsy is a relatively newsurgical technique that is a minimally invasive image-guided
procedure, requiring a small incision that produces a barely noticeable scar. The technique is a useful method for the surgical biopsy of properly selected patients. We reviewed the pathology of the biopsies for the proper selection of a mammotome biopsy in patients with re-excised breast tumors.
Methods
During a 24-month period, we performed vacuumassisted breast biopsies for 277 likely benign breast lesions
using ultrasound and fine-needle aspiration cytology or a core needle biopsy, in 203 patients. The age of the patients ranged from 15 to 67 yr (average age 36.6 yr), and the average size of the lesions was 2.39±1.06 cm (minimum size 0.5 cm, maximum size 5.0 cm). We retrospectively analyzed the pathological findings of the re-excised breast lesions.
Results
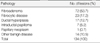
The pathology of ultrasound-guided vacuum biopsies of the benign-appearing breast lesions were fibroadenomas
(69.7%), intraductal papillomas (6.1%), fibrocystic disease (7.9%), phyllodes tumors (2.9%), malignant tumors (1.4%), ductal hyperplasia (2.9%), and other benign diseases (9.1%). Re-excision by a conventional method was performed for nine patients. Reasons for re-excision were the presence of five proven malignancies (a malignant phyllodes tumor in 2 cases, a tubular carcinoma in 1 case, a papillary carcinoma in 1 case and a ductal carcinoma in situ [DCIS] in 1 case), a possible atypical ductal hyperplasia (ADH) malignancy, two marginal involvement in phyllodes tumors and the possible extension of a lesion as an atypical papilloma. In the re-excised specimens, residual tissues were noticed in eight cases. An ADH lesion was proven as a DCIS.
Conclusion
A case of suggested marginal involvements and/or a possible malignancy should be re-excised because of the high possibility of remnant lesions being present after the mammotome biopsy. The cytological and pathological review must be performed precisely before performing the mammotome procedures with considering of the clinical and
radiological findings.
Figures and Tables
References
1. Greenberg R, Skornick Y, Kaplan O. Management of breast fibroa denomas. J Gen Intern Med. 1998. 13:640–645.
3. Fornage BD, Faroux MJ, Simatos A. Breast masses: US-guided fine-needle aspiration biopsy. Radiology. 1987. 162:409–414.

4. Sneige N, Fornage BD, Saleh G. Ultrasound-guided fine-needle aspiration of nonpalpable breast lesion. Cystology and histologic findings. Am J Clin Pathol. 1994. 102:98–101.

5. Parker SH, Klaus AJ. Performing a breast biopsy with a directional, vacuum-assisted biopsy instrument. Radiographics. 1997. 17:1233–1252.

6. Liberman L, Smolkin JH, Dershaw DD, Morris EA, Abramson AF, Rosen PP. Calcification retrieval at stereotactic, 11-gauge, directional, vacuum-assisted breast biopsy. Radiology. 1998. 208:251–260.

7. Liberman L, Vuolo M, Dershaw DD, Morris EA, Abramson AF, LaTrenta LR, et al. Epithelial displacement after stereotactic 11-gauge directional vacuum-assisted breast biopsy. AJR Am J Roentgenol. 1999. 172:677–681.

8. Burak WE Jr, Owens KE, Tighe MB, Kemp L, Dinges SA, Hitchcock CL, et al. Vacuum-assisted stereotactic breast biopsy: histologic underestimation of malignant lesions. Arch Surg. 2000. 135:700–703.
9. Hung WK, Lam HS, Lau Y, Chan CM, Yip AW. Diagnostic accuracy of vacuum-assisted biopsy device for imagedetected breast lesions. Aust N Z J Surg. 2001. 71:457–460.

10. Crowe JP Jr, Rim A, Patrick R, Rybicki L, Grundfest S, Kim J, et al. A prospective review of the decline of excisional breast biopsy. Am J Surg. 2002. 184:353–355.

11. National Library of Medicine. MEDLINEplus health information: fibrocystic breast disease. Accessed on October 14, 2007. Available at:
http://www.nlm.nih.gov/medlineplus/ency/article/000912.htm.
12. Masood S. Cytomorphology of fibrocystic change, high-risk proliferative breast disease, and premalignant breast lesions. Clin Lab Med. 2005. 25:713–731.

13. Smallwood JA, Roberts A, Guyer DP, Taylor I. The natural history of fibroadenoma. Br J Clin Pathol. 1991. 95:614–622.
14. Pick PW, Lossifide IA. Occurrence of breast carcinomas within a fibroadenoma: a review. Arch Pathol Lab Med. 1984. 108:590–593.
15. Krishnamurthy S, Ashfaq R, Shin HJ, Sneige N. Distinction of phyllodes tumor from fibroadenoma: a reappraisal of an old problem. Cancer. 2000. 90:342–349.
16. Chaiwun B, Thorner P. Fine needle aspiration for evaluation of breast masses. Curr Opin Obstet Gynecol. 2007. 19:48–55.

17. White RR, Halperin TJ, Olson JA Jr, Soo MS, Bentley RC, Seigler HF. Impact of core-needle breast biopsy on the surgical management of mammographic abnormalities. Ann Surg. 2001. 233:769–777.

18. Yelland A, Graham MD, Trott PA, Ford HT, Coombes RC, Gazet JC, et al. Diagnosing breast carcinoma in young women. BMJ. 1991. 302:618–620.

19. Obi Iwuagwu, Philip Drew. Vacuum-assisted biopsy device-diagnostic and therapeutic applications in breast surgery. Breast. 2004. 13:483–487.

20. Parker SH, Klaus AJ, McWey PJ, Schilling KJ, Cupples TE, Duchesne N, et al. Sonographically guided directional vacuum-assisted breast biopsy using a hand held device. AJR Am J Roentgenol. 2001. 177:405–408.

21. Brenner RJ. Lesions entirely removed during stereotactic biopsy. Preoperative localization on the basis of mammographic landmarks and feasibility of freehand technique: initial experience. Radiology. 2000. 214:585–590.

22. Kim DY, Lee BC, Jang SY, Ryu JK, Park SY, Kim JK, et al. Usefulness of ultrasound guided vacuum-assisted mammotoem biopsy for breast lesion. J Korean Surg Soc. 2003. 64:109–114.
23. Baez E, Huber A, Vetter M, Hackeroer BJ. Minimal invasive complete excion of benign breast tumors using a three-dimensional US-guided Mammotome vacuum device. US OBGY. 2003. 21:267–272.

24. Fine RE, Israel PZ, Walker LC, Corgan KR, Greenwald LV, Berenson JE, et al. A prospective study of the removal rate of imaged breast lesions by an 11-gauge vacuum assisted biopsy probe system. Am J Surg. 2001. 182:335–400.
25. Parker SH. Sonographically guided directional vacuumassisted breast biopsy using a handheld device. AJR Am J Roentgenol. 2001. 177:405–408.

26. March DE, Coughlin BF, Barham RB. Breast masses: removal of all US evidence during biopsy by using a handheld vacuum assisted device-initial experience. Radiology. 2003. 227:549–555.

27. Park HR, Kwak JY, Jung HK, Lee SH, Shin JY, Kim JU, et al. Is mammotome excision feasible for benign breast mass bigger than 3cm in greatest dimension? J Korean Surg Soc. 2006. 70:25–29.
28. Pae JE, Williams JE. The radiologic features of phyllodes tumor of the breast with clinico-patholgical correlation. Clin Radiol. 1991. 44:8–12.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download