Abstract
Purpose
We aimed to assess the concordance of the immunohistochemical profiles of core biopsy before administrating neoadjuvant chemotherapy with that of the surgical specimens after a definitive operation for breast cancer.
Methods
We retrospectively reviewed the estrogen receptor (ER), progesterone receptor (PR), and HER-2 expressions in 130 consecutive patients who received neoadjuvant chemotherapy and were followed by surgery during the period between February 2002 and March 2006. The pathologic complete tumor response rate for this group was 4.6% (6/130). Both the pre- and post-operative immunohistochemical profiles were available in 32 of the 124 patients (25.8%). Immunohistochemical staining was done on the core biopsies before chemotherapy and on the surgical specimens after operation.
Results
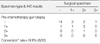
There were 12 markers from 11 patients that were altered out of the 96 total markers (ER, PR, or HER-2) from 32 patients: 2 ER (2/12, 16.7%), 4 PR (4/12, 33.3%), and 6 HER-2 (6/12, 50.0%). One patient simultaneously had changes in the expressions of PR and HER-2. Conversion of the hormone receptor status occurred in 3 patients (3/32, 9.4%): this was positive to negative in two, and vice versa in one. In addition, there were 6 conversions (6/32, 18.8%) of the HER-2 status from negative to positive.
Conclusion
The hormone receptor status changed in 9.4% of the 32 patients and the HER-2 status changed in 18.8% of the 32 patients after neoadjuvant chemotherapy. We have concluded that conducting only a single immunohistochemical study about ER, PR, and HER-2 may not be enough to exactly estimate the tumor marker status in the neoadjuvant setting.
Figures and Tables
Table 5
Clinicopathologic characteristics of 11 female patients who experienced receptor expression changes after neoadjuvant chemotherapy

Pt=patient's number; Dx=histologic diagnosis; NG=nuclear grade; HG=histologic grade; CTx=chemotherapy; Op=operation type; ER=estrogen receptor; PR, progesterone receptor; IDC=infiltrating ductal carcinoma; MC=medullary carcinoma; IMC=invasive micropapillary carcinoma; PR=partial response; SD=stable disease; PD=progressive disease; BCS=breast conserving surgery; MRM=modified radical mastectomy; NA=not available.
*One patient (pt 3) simultaneously experienced PR down-expression and HER-2 up-expression.
References
1. Lee SH, Chung MA, Quddus MR, Steinhoff MM, Cady B. The effect of neo-adjuvant chemotherapy on estrogen and progesterone receptor expression and hormone receptor status in breast cancer. Am J Surg. 2003. 186:348–350.

2. Piper GL, Patel NA, Patel JA, Malay MB, Julian TB. Neoadjuvant chemotherapy for locally advanced breast cancer results in alterations in preoperative tu-mor marker status. Am Surg. 2004. 70:1103–1106.
3. Taucher S, Rudas M, Gnant M, Thomanek K, Dubsky P, Roka S, et al. Sequential steroid hormone receptor measurements in primary breast cancer with and without intervening primary chemotherapy. Endocr Relat Cancer. 2003. 10:91–98.

4. Mann GB, Fahey VD, Feleppa F, Buchanan MR. Reliance on hormone receptor assays of surgical specimens may compromise outcome in patients with breast cancer. J Clin Oncol. 2005. 23:5148–5154.

5. Arens N, Bleyl U, Hildenbrand R. HER2/neu, p53, Ki67, and hormone receptors do not change during neoadjuvant chemotherapy in breast cancer. Virchows Arch. 2005. 446:489–496.

6. Varga Z, Caduff R, Pestalozzi B. Stability of the HER2 gene after primary chemotherapy in advanced breast cancer. Virchows Arch. 2005. 446:136–141.

7. Taucher S, Rudas M, Mader RM, Gnant M, Sporn E, Dubsky P, et al. Influence of neoadjuvant therapy with epirubicin and docetaxel on the expression of HER2/neu in patients with breast cancer. Breast Cancer Res Treat. 2003. 82:207–213.

8. Lebeau A, Deimling D, Kaltz C, Sendelhofert A, Iff A, Luthardt B, et al. HER-2 analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol. 2001. 19:354–363.

9. Perez EA, Roche PC, Jenkins RB, Reynolds CA, Halling KC, Ingle JN, et al. HER2 testing in patients with breast cancer: poor correlation between weak positivity by immunohistochemistry and gene amplification by fluorescence in situ hybridization. Mayo Clin Proc. 2002. 77:148–154.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download