Abstract
Purpose
The behavior of invasive carcinomas in human can be very varied with different individual responses to chemotherapy. Individualization is crucial to the optimization of chemotherapy. Therefore, the prediction of a tumor's sensitivity to anticancer agents has been the subject of intensive investigation. In order to investigate the pathobiology of breast cancer, it is necessary to maintain or recreate the characteristics of the three-dimensional architecture of the tissues in culture. In this study, we have evaluated the relationship between the Histoculture Drug Response Assay (HDRA) assessment and chemotherapy responses in breast cancer patients.
Methods
Tumor specimens from 30 patients with breast cancer were evaluated using the HDRA. Tumor tissues were cultured on gelfoam sponge gel in 24-well plates, followed by treatment with a variety of chemotherapeutic agents. All treatments were conducted in triplicate. The sensitivity of a chemotherapy regimen was defined as a tumor inhibition ate (IR) in excess of 30%. Neoadjuvant or palliative chemotherapy for patients, using anthracycline or taxane, was conducted on the basis of the established protocols. The responses to treatments were compared with the results of the HDRA.
Results
The mean IR for the combinations of doxorubicin and docetaxel and for FAC and AC were 48, 45, and 36%, respectively. The above partial rate of response to chemotherapy was 81.1%. The sensitivity and specificity of the HDRA assessment, with a 30% inhibition rate, were 81.5 and 66.7%, respectively. The positive and negative response prediction values were 91.7 and 44.4%, respectively. The responses to treatments and the results of the HDRA assessment were not correlated with the expressions of the hormonal receptor or c-erbB2.
Conclusion
In cases in which the inhibition rate is in excess of 30%, the HDRA assessment yielded a high positive response prediction value. The sensitivity to chemotherapy, as determined by the HDRA, appears to be a good guide for selection in breast cancer patients. Thus the results presented herein should be integrated into future research on the subject.
Figures and Tables
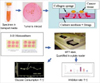 | Fig 2Average inhibiton rate in 3-dimensional histoculture drug response assay.
ADR=adriamycin; CTX=cyclophosphamide; FAC=combination of 5FU, adriamycin, cyclophosphamide; AC=combination of adriamycin, cyclophosphamide; AD=combination of adriamycin, docetaxel.
|
References
1. Kern DH, Wiesenthal LM. Highly specific prediction of antineoplastic drug resistance with an in vitro assay using suprapharmacologic drug exposure. J Natl Cancer Inst. 1990. 82:582–588.

2. Hoffman RM. In vitro assays for chemotherapy sensitivity. Crit Rev Oncol Hematol. 1993. 15:99–111.

3. Kim JB, Stein R, O'Hare MJ. Three-dimensional in vitro tissue culture models of breast cancer-a review. Breast Cancer Res Treat. 2004. 85:281–291.

4. Furukawa T, Kubota T, Hoffman RM. Clinical applications of the histoculture drug response assay. Clin Cancer Res. 1995. 1:305–311.
5. Furukawa T, Kubota T, Watanabe M, Takahara T, Yamaguchi H, Takeuchi T, et al. High in vitro-in vivo correlation of drug response using sponge-gel-supported three-dimensional histoculture and the MTT end point. Int J Cancer. 1992. 51:489–498.

6. Hoffman RM. To do tissue culture in two or three dimensions? that is the question. Stem Cells. 1993. 11:105–111.

7. Sourla A, Doillon C, Koutsilieris M. Three-dimensional type I collagen gel system containing MG-63 osteoblasts-like cells as a model for studying local bone reaction caused by metastatic cancer cells. Anticancer Res. 1996. 16:2773–2780.
8. Jacquot J, Spilmont C, Burlet H, Fuchey C, Buisson AC, Tournier JM, et al. Glandular-like morphogenesis and secretory activity of human tracheal gland cells in a three-dimensional collagen gel matrix. J Cell Physiol. 1994. 161:407–418.

9. Kang HJ, Ko CD, Yoon HS, Kim MB, Ahn SH. The reliability of histoculture drug response assay (HDRA) in chemosensitivity tests for breast cancer. Cancer Res Treat. 2001. 33:392–398.

10. Vescio RA, Connors KM, Kubota T, Hoffman RM. Correlation of histology and drug response of human tumors grown in native-state three-dimensional histoculture and in nude mice. Proc Natl Acad Sci USA. 1991. 88:5163–5166.

11. Singh B, Li R, Xu L, Poluri A, Pastel S, Shaha AR, et al. Prediction of survival in patients with head and neck cancer using the histoculture drug response assay. Head Neck. 2002. 24:437–442.

12. Yoshimasu T, Oura S, Maebeya S, Tanino H, Bessho T, Arimoto J, et al. Histoculture drug response assay on non-small cell lung cancer. Gan To Kagaku Ryoho. 2000. 27:717–722.
13. Ariyoshi Y, Shimahar M, Tanigawa N. Study on chemosensitivity of oral squamous cell carcinomas by histoculture drug respose assay. Oral Oncol. 2003. 39:701–707.

14. Hirano Y, Ushiyama T, Suzuki K, Fujita K. Clinical application of an in vitro chemosensitivity test, the Histoculture Drug Response Assay, to urological cancers: wide distribution of inhibition rates in bladder cancer and renal cell cancer. Urol Res. 1999. 27:483–488.

15. Ohie S, Udagawa Y, Kozu A, Komuro Y, Aoki D, Nozawa S, et al. Cisplatin sensitivity of ovarian cancer in the histoculture drug response assay correlates to clinical response to combination chemotherapy with cisplatin, doxorubicin and cyclophosphamide. Anticancer Res. 2000. 20:2049–2054.




 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download