Abstract
Purpose
Published Early Breast Cancer Trialists' Collaborative Group overview results have been the beneficial effects of tamoxifen and ovarian ablation for pre and perimenopausal women with node negative breast cancer. Chemotherapy and Luteinizing Hormone Releasing Hormone (LHRH) agonists (medical ovarian ablation) have been shown to be effective adjuvant therapies for early stage breast cancer in several clinical trials however, the efficacy and tolerance of LHRH agonists in Korean breast cancer patients has not been evaluated.
Methods
Three thousand one hundred fifty breast cancer patients were treated at Asan Medical Center between January 2003 and December 2005. We selected 185 patients with node negative early breast cancer who were endocrine responsive (more than intermediate intensity), with a tumor size more than 1 cm, and who were reluctant to undergo chemotherapy due to the side effects. They received LHRH agonists (Zoladex® 3.6 mg) every 28 days with tamoxifen for two years. We prospectively evaluated mammography, chest PA, and physical examination every six months and evaluated the side effects and quality of life.
Results
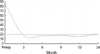
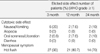
The mean age was 43.5 yr, and the mean tumor size was 1.62 cm. One hundred sixty-two patients had Stage I, and 23 Stage II disease. The incidence of severe menopausal symptoms was 24.1%, but these symptoms were reported to be "tolerable" during the two year follow-up. Quality of life and physical activity were essentially unchanged. The median follow-up duration was 18 months, and there was no local recurrence or distant metastases during the study.
References
1. Son BH, Kwack BS, Kim JK, Kim HJ, Hong SJ, Lee JS, et al. Changing patterns in the clinical charateristics of Korean Patients with breast cancer during the last 15 years. Arch Surg. 2006. 141:155–160.

2. Korean Breast. Nationwide Korean Breast Cancer Data of 2002. J Korean Breast Cancer Soc. 2004. 7:72–83.

3. Ahn SH. Korean Breast Cancer Society. Clinical Charateristics of breast cancer patient in Korea in 2000. Arch Surg. 2004. 139:27–30.
4. Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting Highlights: International Expert Consensus on the primary therapy of early breast caner. Ann Oncol. 2005. 16:1569–1583.

5. Miller AB, Hoogstraten B, Staquwt M, Winkler A. Reporting results of cancer treatment. Cancer. 1981. 47:207–214.

6. Early Breast. Effect of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival; an overview if the randomized trials. Lancet. 2005. 365:1687–1717.
7. Early Breast Cancer Trialists' Collaborative Group. Polychemotherapy for early breast cancer: an overview of the randomized trials. Lancet. 1998. 352:930–942.
8. Jonat W, Kaufmann M, Sauerbrei W, Blamey R, Cuzick J, Namer M, et al. Goserelin versus cyclophosphamide, methotrexate and flurouracil as adjuvant therapy in premenopausal patients with node-positive breast cancer: The Zoladex Early Breast Cancer Research Association. J Clin Oncol. 2002. 20:4628–4635.

9. International Breast Cancer Study Group. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast caner: a randomozied trial. J Natl Cancer Inst. 2003. 95:1833–1846.
10. National Institutes of Health Consensus Developement Conference statement. Adjuvant therapy for breast caner. 2000. J Natl Cancer Inst Monogor. 2001. 93:979–989.
11. Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thurlimann B, Senn HJ. Meeting highlights: updated international expert consensus on the primary therapy of early breast cancer. J Clin Oncol. 2003. 21:3357–3365.

12. Williams MR, Walker KJ, Turkes A, Blamey RW, Nicholsen RI. The use of anjHRH agonist in advanced premenopausal breast cancer. Br J Cancer. 1986. 53:629–636.

13. Jonat W. Zoladex versus CMF adjuvant therapy in pre/perimenopausal breast cancer:tolerability and amenorrhea comparisions. Pro Am Soc Clin Oncol. 2000. 19:87.
14. Wallwiener D, Possinger K, Bonder G, Schmid P, Untch M, Kosse V, et al. Leuprorelin acetate vs. CMF in the adjuvant treatment of premenopausal women with ER/PR- positive, node-positive breast cancer: interim results of the TABLE study. Pro Am Soc Clin Oncol. 2001. 20:34a.
15. Klijin J, de Jong F. Treatment with a leuteinaising-hormone releasing hormone analogue (buserelin) in premonopausal patients with metastatic breast cancer. Lancet. 1982. 1:1213–1216.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download