Abstract
Objectives
To determine the proteoglycan synthesis of the rabbit nucleus pulposus cells in various concentration of extracellular collagen type I and II under the stimulation of TGF- β1.
Summary of Literature Review
Therapeutic effect of growth factor and gene therapy can be altered by composition of extracellular matrix. However, the effect of extracellular collagen types I and II on synthetic activity of intervertebral disc cells is not thoroughly studied before.
Materials and Methods
The nucleus pulposus cells were isolated and cultured from 10 skeletally mature rabbits. Cultures were trypsinized and incorporated into alginate beads with different concentration of extracellular collagen type I and II (0.5%, 1.0% and 1.5%). Those cultures with TGF- β1 (10 ng/ml) served stimulated condition of matrix synthesis. Newly synthesized proteoglycans were assessed by 35 S- sulfate incorporation using chromatography on Sephadex G- 25 in PD- 10 columns. Scintillation count was normalized with DNA content by Hoechst dye method.
Results
In basal condition, difference in proteoglycan synthesis in given concentration of extracellular collagen type I and II were statistically insignificant. In stimulated condition with TGF- β1, difference in proteoglycan synthesis in given concentration of extracellular collagen type I and II was also statistically insignificant. However, cultures in stimulated condition with TGF- β1 showed increased amount of newly synthesized proteoglycans compared to those of basal condition regardless of the concentration of extracellular collagen type I and II (p <0.05).
REFERENCES
1). Anderson JAD. Back pain and occupation. Jayson MIV, editor. The lumbar Spine and Back Pain.3rd ed. London: Chirchill Livingstone;p. 2–36. 1987.
2). Antoniou J, Steffen T, Nelson F, et al. The human lumbar vertebral disc: evidence of changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 98:996–1003. 1996.
3). Ballock RT, Heydemann A, Izumi T, Reddi AH. Regulation of the expression of the type-II collagen gene in periosteum-derived cells by three members of the transforming growth factor-beta superfamily. J Orthop Res. 15:463–467. 1997.
4). Benya PD, Shaffer JD. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 30:215–224. 1982.

5). Borenstein D. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr Opin Rheumatol. 4:226–232. 1992.

6). Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 20:1307–1314. 1995.

7). Buckwalter JA, Pedrini-Mille A, Pedrini V, Tudis-co C. Proteoglycans of human infant intervertebral disc: electron microscopic and biochemical studies. J Bone Joint Surg. 67-A:284–294. 1985.

8). Butler D, Trafimow JH, Andersson GB, McNeill TW, Huckman MD. Discs degenerate before facets. Spine. 15:111–113. 1990.

9). Gan JC, Ducheyne P, Vresilovic E, Shapiro IM. Bioactive glass serves as a substrate for maintenance of phenotype of nucleus pulposus cells of the intervertebral disc. J Biomed Mater Res. 51:596–604. 2000.

10). Gruber HE, Fisher EC Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN Jr. Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-β 1. Exp Cell Res. 235:13–21. 1997.
11). Hayes AJ, Benjamin A, Ralphs JR. Role of actin stress fibers in the development of the Intervertebral disc: cytoskeletal control of extracellular matrix assembly. Dev Dyn. 215:179–189. 1999.
12). Hutton WC, Elmer WA, Boden SD, Horton WC, Carr K. Analysis of chondroitin sulfate in the lumbar intervertebral discs at two different stages of degeneration as assayed by discogram. J Spinal Disord. 10:47–54. 1997.
13). Ichimura K, Tsuji H, Matsui H, Makiyama N. Cell culture of the Intervertebral disc of rats: factors influencing culture, proteoglycan, collagen, and deoxyribonucleic acid synthesis. J Spinal Disord. 4:428–436. 1991.
14). Ingber DE, Folkman J. Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in vitro: role of extracellular matrix. J Cell Biol. 109:317–330. 1989.

15). Inkinen RI, Lammi MJ, Lehmonen S, Puustjarvi K, Kaapa E, Tammi MI. Relative increase of biglycan and decorin and altered chondroitin sulfate epitopes in the degenerating human intervertebral disc. J Rheumatol. 25:506–514. 1998.
16). Itay S, Abramovici A, Nevo Z. Use of cultured embryonal chick epiphyseal chondrocytes as grafts for defects in chick articular cartilage. Clin Orthop. 220:284–303. 1987.

17). Kim BS, Jahng JS. Change of the effect of TGF-β 1 on physeal chondrocytes according to culture methods in vitro. J of Korean Orthop Assoc. 34:849–857. 1999.
18). Konttinen YT, Kemppinen P, Li TF, et al. Transforming and epidermal growth factors in degenerated intervertebral discs. J Bone Joint Surg. 81-B:1058–1063. 1999.

19). Lee JW, Kim NH. Change of type I and type II collagen biosynthesis by growth factors in cultured cells isolated from rabbit intervertebral disc. J of Korean Orthop Assoc. 33:1867–1882. 1998.
20). Lehmann TR, Spratt KF, Tozzi JE, et al. Long-t erm followup of lower lumbar fusion patients. Spine. 12:97–104. 1987.
21). Lipson SJ, Muir H. Proteoglycans in experimental intervertebral disc degeneration. Spine. 6:194–210. 1981.

22). Luyten FP, Hascall VC, Nisseley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 267:416–425. 1988.
23). Maldonado BA, Oegema TR Jr. Initial characteri -zation of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 10:677–690. 1992.
24). Mankin HJ. Localization of tritiated thymidine in articular cartilage of rabbits. J Bone Joint Surg. 44-A:688–698. 1962.

25). Morales TI. Transforming growth factor-beta and insulin-like growth factor-1 restore proteoglycan metabolism of bovine articular cartilage after depletion by retinoic acid. Arch Biochem Biophys. 315:190–198. 1994.
26). Morales TI, Roberts AB. Transforming growth factor beta regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 263:12828–12831. 1988.

28). Nishida K, Gilbertson LG, Robbins PD, Evans CH, Kang JD. Potential applications of gene therapy to the treatment of intervertebral disc disorders. Clin Orthop. 379(Suppl):234–241. 2000.

29). Nishida K, Kang JD, Gilbertson LG, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 24:2419–2425. 1999.
30). O’ Driscoll SW, Keeley FW, Salter RB. Durability of regenerated articular cartilage produced by free auto -genous periosteal grafts in major full-thickness defects in joint surfaces under the influence of continuous passive motion. a followup report at one year. J Bone Joint Surg. 70-A:595–606. 1988.
31). Qi WN, Scully SP. Effect of type II collagen in chondrocyte response to TGF-β 1 regulation. Exp Cell Res. 241:142–150. 1998.
32). Qi WN, Scully SP. Extracellular collagen modulates the regulation of chondrocytes by transforming growth factor-beta1. J Orthop Res. 15:483–490. 1997.
33). Robinson D, Mirovsky Y, Halperin N, Evron Z, Nevo Z. Changes in proteoglycans of intervertebral disc in diabetic patients: a possible cause of increased back pain. Spine. 23:849–856. 1998.
34). Shinmei M, Kikuchi T, Yamagishi M, Shimomura Y. The role of interleukin-1 on proteoglycan metabolism of rabbit annulus fibrosus cells cultured in vitro. Spine. 13:1284–1290. 1988.
35). Shinmei M, Masuda K, Kikuchi T, Shimomura Y. The role of cytokines in chondrocyte mediated cartilage degradation. J Rheumatol, (Suppl 18). 16:32–34. 1989.
36). Sporn MB, Roberts AB. Transforming growth factor -β: recent progress and new challenges. J Cell Biol. 119:1017–1021. 1992.
37). Thompson JP, Oegema TR, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 16:253–260. 1991.

38). Trippel SB, Wroblewski J, Makower AM, Whelan MC, Schoenfeld D, Doctrow SR. Regulation of growth-plate chondrocytes by insulin-like growth-factor I and basic fibroblast growth factor. J Bone Joint Surg. 75-A:177–189. 1993.

39). Vacanti CA, Langer R, Schloo B, Vacanti JP. Synthetic polymers seeded with chondrocytes provide a tem -plate for new cartilage formation. Plast Reconstr Surg. 88:753–759. 1991.
40). Wakitani S, Kimura T, Hirooka A, et al. Repair of rabbit articular surfaces with allograft chondrocytes embed -ded in collagen gel. J Bone Joint Surg. 71-B:74–80. 1989.
Fig. 1.
The effect of type I collagen on the synthesis of proteoglycan by rabbit disc cells encapsulated in alginate beads. Incorporation of 35S-sulfate into disc cells cultured in basal medium or in medium supplemented with transforming growth factor-β1 (TGF-β1) was measured. Data represent mean ± SD of three experiments performed in triplicate. The values of Y-axis were content of newly synthesized proteoglycan, which was divided by the amount of deoxyribonucleic acid. Rabbit intervertebral disc cells cultured in alginate beads, treated by TGF-β1 (10 ng/ml) showed 3 fold increase in newly synthesized proteoglycan compared to basal condition (p<0.05), while increased concentration of extracellular type I collagen failed to affect proteoglycan synthesis (p>0.05). *: p<0.05, NS: statistically non-significant.
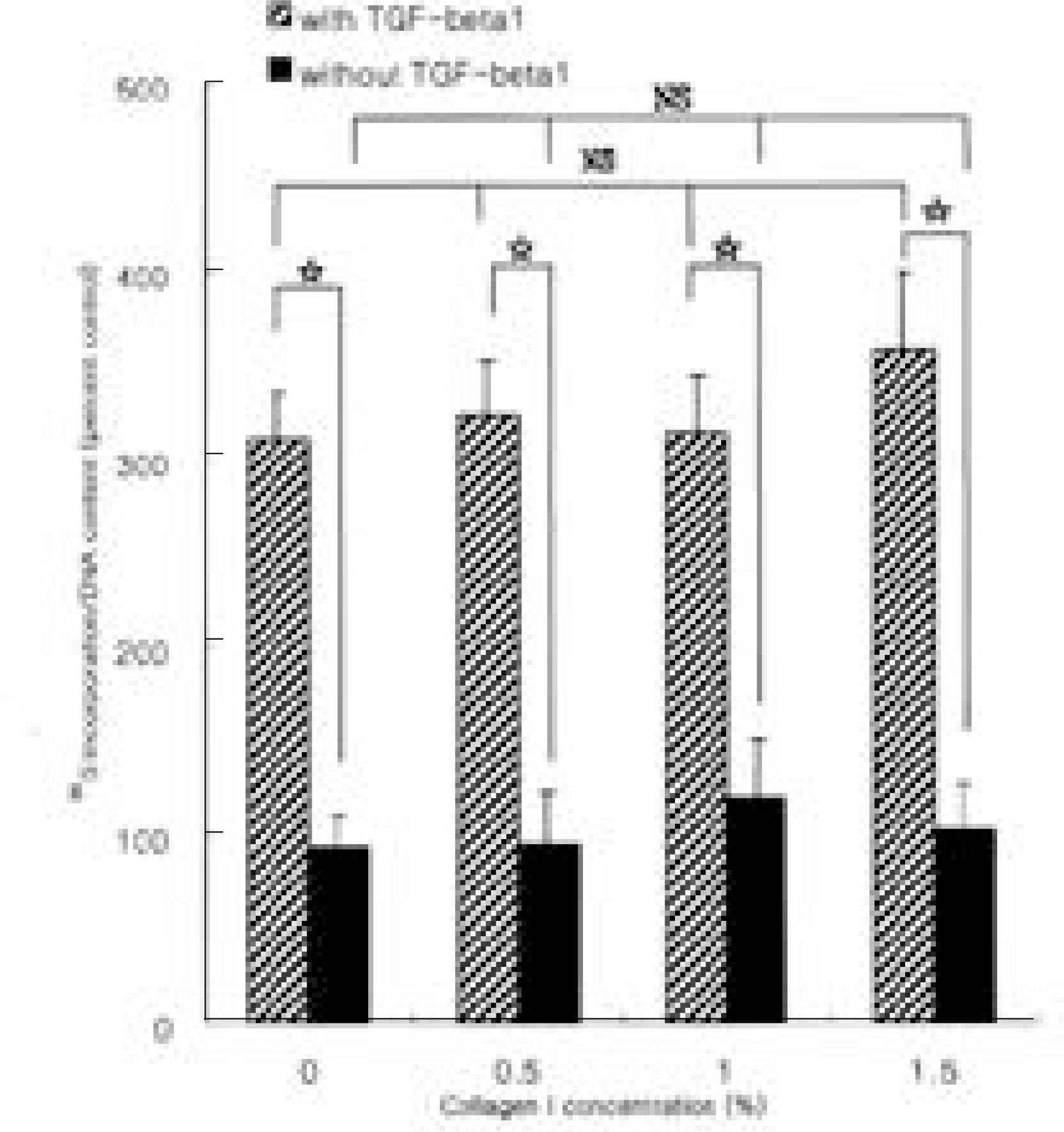
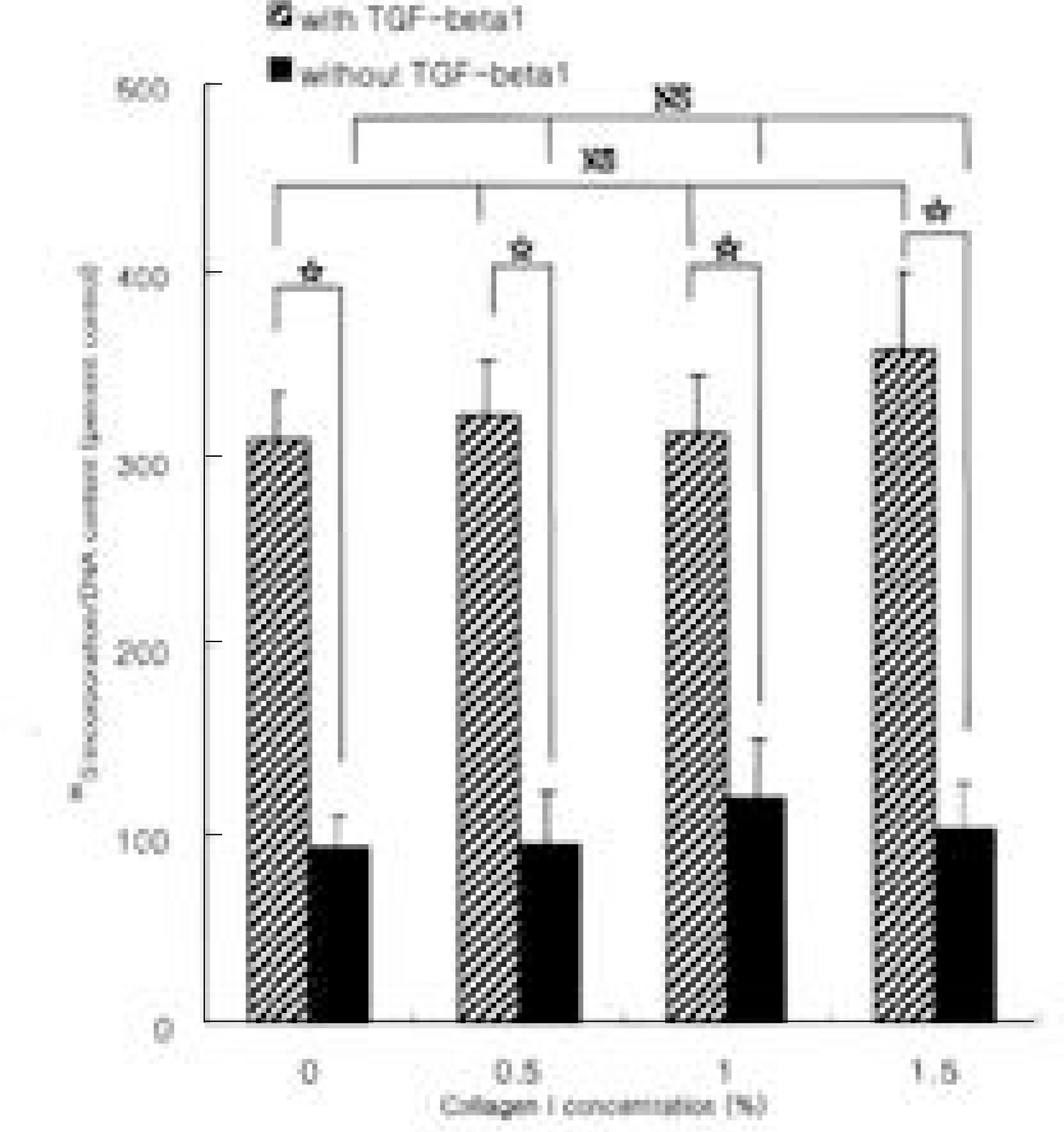
Fig. 2.
The effect of type II collagen on the synthesis of proteoglycan by rabbit disc cells encapsulated in alginate beads. Incorporation of 35S-sulfate into disc cells cultured in basal medium or in medium supplemented with transforming growth factor-β1 (TGF-β1) was measured. Data represent mean ± SD of three experiments performed in triplicate. The values of Y-axis were content of newly synthesized proteoglycan, which was divided by the amount of deoxyribonucleic acid. Rabbit intervertebral disc cells cultured in alginate beads, treated by T GF-β1 (10 ng/ml) showed 3 fold increase in newly synthesized proteoglycan compared to basal condition (p<0.05), while increased concentration of extracellular type II collagen failed to affect proteoglycan synthesis (p>0.05). *: p<0.05, NS: statistically non-significant.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download