Abstract
Study Design
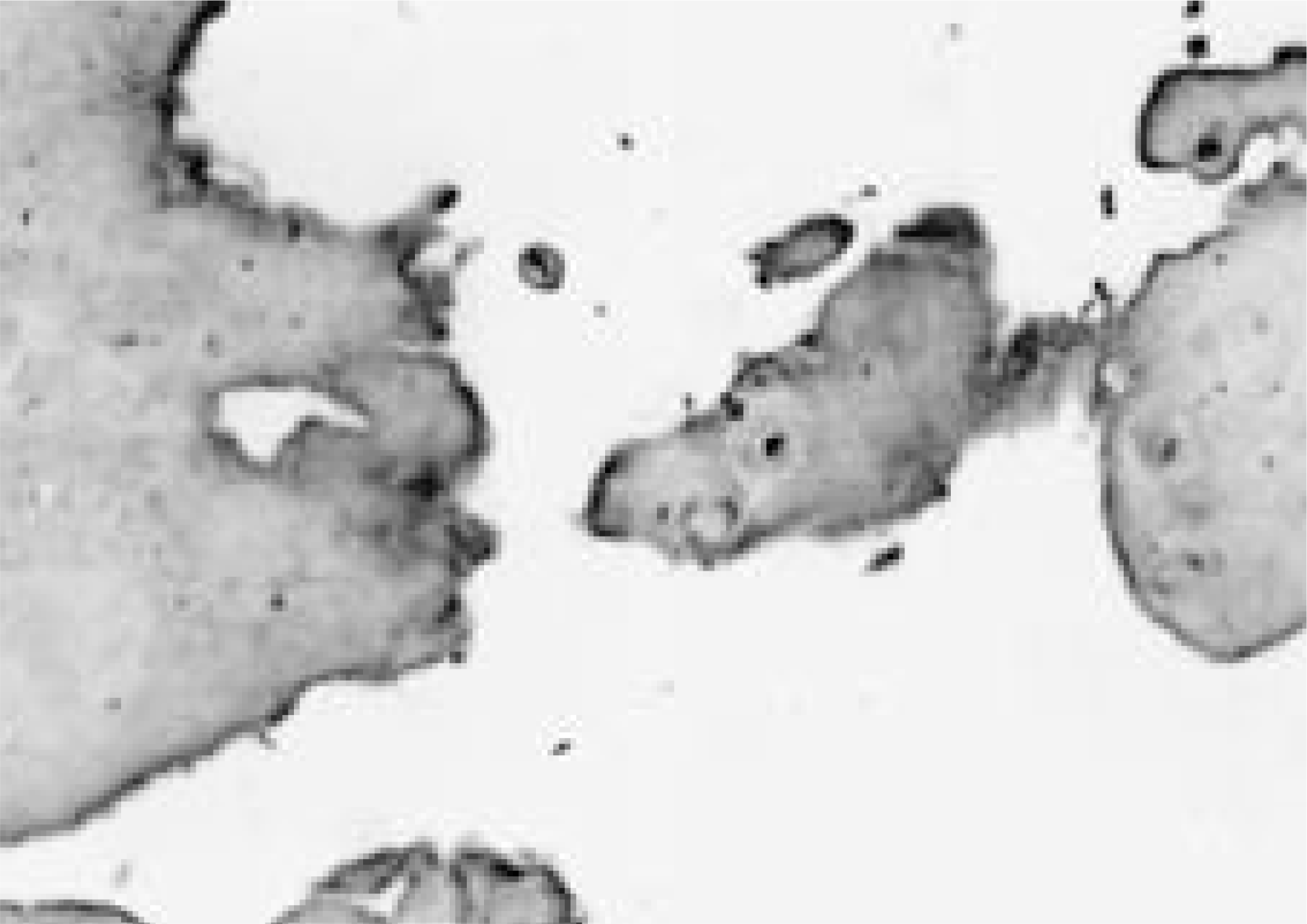
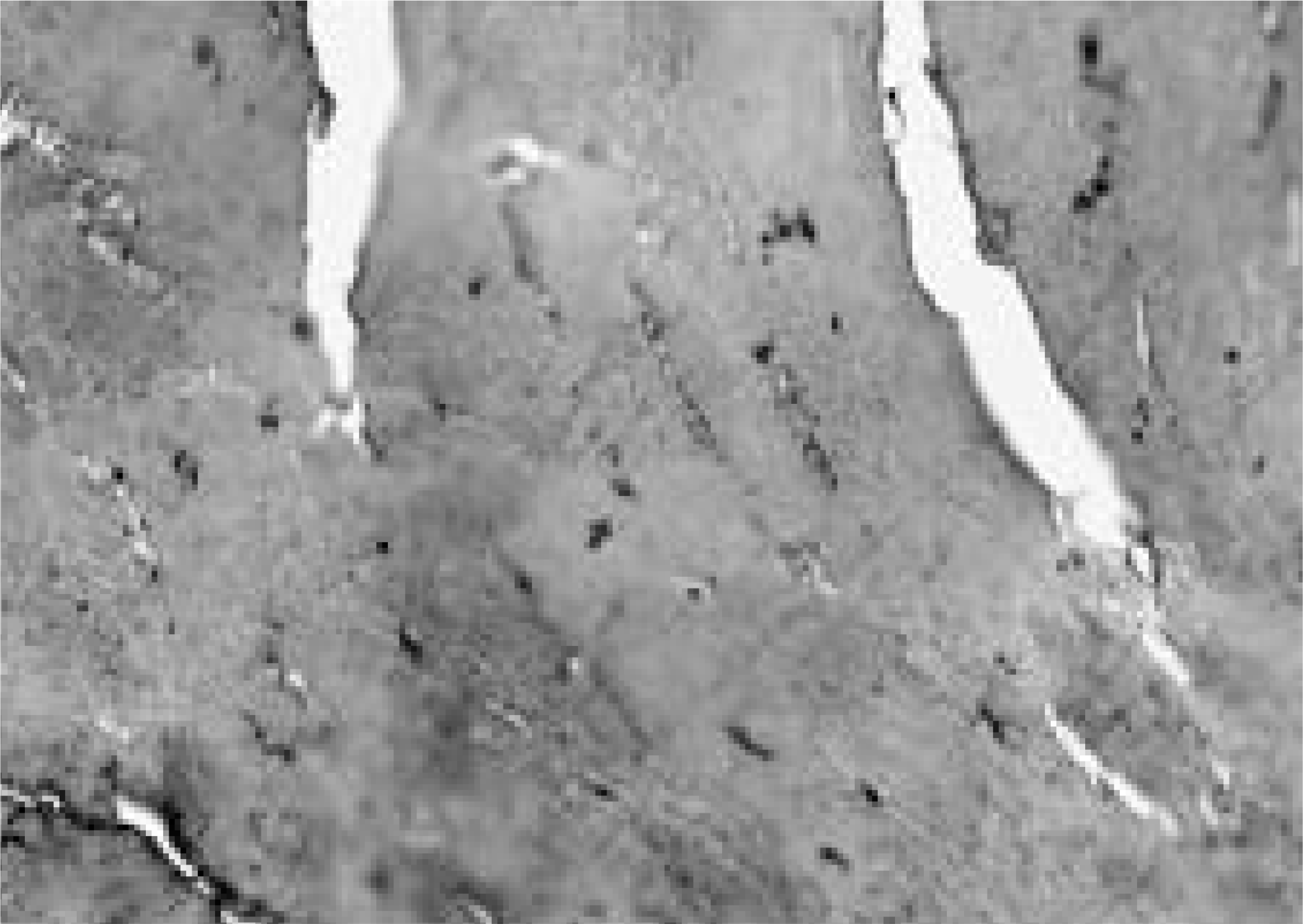
Immunohistologic staining of Intervertebral discs at surgery was performed using CD68, matrix metallopro-teinase- 1(MMP- 1) and matrix metalloproteinase- 3(MMP- 3).
Objectives
1. To investigate the histologic characteristics of the macrophage infiltrations in herniated discs and degenerative discs. 2. To investigate the difference of immumoexpression of MMP- 1 and MMP- 3 activity to herniated discs and degenerative discs. 3. To investigate the possible correlation between the macrophage infiltrations, MMP- 1and MMP- 3 in herniated discs and degenerative discs.
Summary of Literature Review
Spontaneous absorption of herniated disc is associated with infiltrating macrophages and matrix metalloproteinases. Matrix metalloproteinases(MMP) are main matrix degradative enzymes in intervertebral disc.
Materials and Methods
Sixty- seven specimens of degenerative disc specimens and one hundred and two specimens of lumbar disc herniation were immunostained with monoclonal antibodies to CD68, anti- MMP- 1, and anti- MMP- 3.
Results
The granulation tissue containing many CD68- positive macrophages was commonly observed in herniated disc and not in degenerative disc. The immunoexpression of MMP- 1 and MMP- 3 were observed in herniated and degenerative disc and there were the differences of intensity and localization of MMP- 1and MMP- 3 expression in herniated disc and not in degenerative disc. There was no correlation between the macrophage infiltrations, MMP- 1and MMP- 3 expression.
Go to : 
REFERENCES
2). Cawston TE, Billington C. Metalloproteinases in the rheumatic diseases. J Pathol. 180:115–117. 1996.
3). Chin JR, Murphy G, Werb Z. Stromelysin, a connective tissue-degrading metalloendopeptidase secreted by stimulated rabbit synovial fibroblasts in parallel with collagenase. J Biol Chem. 260:12367–12376. 1985.
4). DiMartino MJ, Wolff CE, High W, Crimmin MJ, Galloway WA. Antiinflammatory and chondroprotective activities of a potent metalloproteinase inhibitor. J Cell Biochem. 19(Suppl E):179. 1991.
5). Doita M, Kanatani T, Harada T, Mizuno K. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine. 21:235–241. 1996.

6). Goupille P, Jayson MIV, Valat JP, Freemont AJ. Matrix Metalloproteinases; The clue to intervertebral disc degenerations? Spine. 23:1612–1626. 1998.
7). Gronblad M, Virri J, Tolonen J, et al. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine. 19:2744–2751. 1994.

8). Gronblad M, Virri J, Tolonen J, Kankare J, Seitsalo S, Karaharju EO. Stromelysin expression in disc herniation cells. Presented at the annual meeting of International Society for the study of the Lumbar Spine, Seattle, Washington, June 21-25. 1995.
9). Haro H, Crawford HC, Fingleton B, et al. Matrix met -alloproteinase-7-dependent release of tumor necrosis factor- in a model of herniated disc resorption. J Clin Invest. 105:143–150. 2000.
10). Haro H, Shinomiya K, Komori H, et al. Upre gulated expression of chemokines in herniated nucleus pulposus resorption. Spine. 21:1647–1652. 1996.
11). Haro H, Shinomiya K, Murakami S, Spengler DM. Up-regulated expression of matrilysin and neutrophil collagenase in human herniated discs. J Spinal Disord. 13:245–249. 1999.

12). Hasty KA, Jeffrey JJ, Hibbs MS, Welgus HG. The collagen substrate specificity of human neutrophil collagenase. J Biol Chem. 262:1048–1052. 1987.

13). Kanemoto M, Hukuda S, Komiya Y, Katsuura A, Nishioka J. Immunohistochemical study of matrix metal -loproteinase-3 and tissue inhibitor of metalloproteinase-1 in human intervertebral discs. Spine. 21:1098–1104. 1996.
14). Komori H, Shinomiya K, Nakai O, Yamaura I, Takeda S, Furuya K. The natural history of herniated nucleus pulposus with radiculopathy. Spine. 21:225–229. 1996.

15). Liu J, Roughley PJ, Mort JS. Identification of human intervertebral disc stromelysin and its involvement in matrix degradation. J Orthop Res. 9:568–575. 1991.

16). Melrose S, Ghosh P, Taylor TKF. Neutral proteinases of the human intervertebral disc. Biochim Biophys Acta. 923:483–495. 1987.

17). Murphy GJP, Murphy G, Reynolds JJ. The origin of matrix metalloproteinases and their family relation -ships. FEBS Lett. 289:4–7. 1991.
18). Nguyen Q, Murphy G, Roughley PJ, Mort JS. Degradation of proteoglycan aggregate by a cartilage metalloproteinase: Evidence for the involvement of stromelysin in the degradation of link protein heterogene -ity in situ. Biochem J. 244:27–33. 1989.
19). Okada Y, Shinmei M, Tanaka O, et al. Localization of matrix metalloproteinase 3 (stromelysin) in osteoarthritic cartilage and synovium. Lab Invest. 66:680–690. 1992.
20). Quantin B, Murphy G, Breathnach R. Pum p -1 cDNA codes for a protein with characteristics similar to those of classical collagenase family members. Biochem -istry. 28:5327–5334. 1989.
21). Sedowofia KA, Tomlinson IW, Weiss JB, Hilton RC, Jayson MIV. Collagenolytic enzyme systems in human intervertebral disc. Their control, mechanism, and their possible role in the initiation of biochemical failure. Spine. 7:213–222. 1982.
22). Thompson JP, Oegema TR, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 16:253–260. 1991.

23). Welgus HG, Jeffrey JJ, Eisen AZ. The collagen substrate specificity of human skin fibroblast collagenase. J Biol Chem. 256:9511–9515. 1981.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download