Abstract
Study design
This is a prospective study to determine whether calcium sulfate(CS) used as a bone graft expander could pro-mote spinal fusion as effectively as autogenous bone graft.
Objectives
To investigate the ability of CS to serve as bone graft substitute when combined in a 1:1 ratio with autogenous graft bone(A GB).
Semmary and Literature Review
A utogenous bone is considered the most successful bone graft material and is presently gold standard. Many complications, however, have been reported. Thus, numerous biodegradable osteoconductive ceramic bone graft substitute have received attention as alternative to autogenous bone to reduce the complications. The advantage of a biodegradable graft material is its compatibility with the new bone remodeling process required to attain optimum mechanical strength.
Materials and Methods
Fifteen patients who had undergone posterolateral spinal fusion with instrumentation using CS mixed with A GB were evaluated. The patients received the autogenous iliac crest graft on one side of the spine and an equivalent vol-ume of autogenous iliac crest/ CS combination on the other side. Thus, the patients serve as their own control. The number of segments fused was 45 segments. The implanted sites were assessed for new bone formation and bony fusion by plain radiog-raphy and CT.
Results
Of 47 segments fused with CS and A GB, 42 segments (89.4%) were completely fused. In contrast, segments fused with A GB alone, 44 segments (93.6%) were fused. One patient showed nonunion at the both side. Two patients had nonunion at the fused segments with CS and A BG. However, the other side showed complete union. 5 patients who underwent removal of hardware had grossly and histologically complete union. There were no complications related to CS.
Go to : 
REFERENCES
1). 하기용, 한창환, 류승준. 가토에서 골 대치물인 calcium sulfate를 이용한 횡돌기간 요추 유합술. 대한척추외과학회지. 6:336–343. 1999.
2). Bell WH. Resorption characteristics of bone and plaster. Oral Surg. 39:727. 1960.
3). Boden SD, Martin GJ, Morone M, et al. The use of coralline hydroxyapatite with bone marrow, autogenous bone graft, or osteoinductive bone protein extract for posterolateral lumbar fusion. Spine. 24:320–327. 1999.
4). Boden SD, Schimandle JH and Hutton WC. Lumba r intertransverse-process spinal arthrodesis with use of a bovine bone-derived osteoinductive protein: A preliminary report. J Bone Joint Surg. 77A:1404–1417. 1995.
5). Bucholz RW, Carlton A and Holmes RZ. Hydroxyapatite and tricalcium phosphate graft substitute. Orthop Clin North Am. 18:323–334. 1987.
6). Coetzee AS. Regeneration of bone in the presence of calcium sulfate. Arch Otolaryngol. 106:405–409. 1980.

7). Delecrin J, Aguado E, Nguyen JM, Pyre D, Royer J and Passuti ZV. Influence of local environment on incorporation of ceramic for lumbar fusion. Comparison of laminar and intertransverse site in a canine model. Spine. 22:1683–1689. 1977.
8). Delecrin J, Takahashi S, Gouin F and Passuti N. A syn -thetic porous ceramic as a bone graft substitute in the surgical management of scoliosis. Spine. 25:563–569. 2000.
9). Ferraro JW. Experimental evaluation of ceramic calcium phosphate as a subititute for bone grafts. Plast Recon -str Surg. 63:634. 1979.
10). Frenkel SR, Moskovich R, Spivak J, et al. Demineral -ized bone matrix: Enhancement of spinal fusion. Spine. 18:1634–1639. 1993.
11). Hadijivavlou AG, Simmons JW, Youg J, Nicodemus CL, Esch O and Simmons DJ. Plaster of Paris as an osteochonductive material for interbody vertebral fusion in mature sheep. Spine. 25:10–16. 2000.
12). Jarcho N. Calcium phosphate ceramics as hard tissue prosthesis. Clin Orthop. 157:259–278. 1981.
13). Lenke LG, Bridwell KH, Baldus C, Shoenecker PL, et al. Results of in-situ fusion for isthmic spondylolisthesis. Federation of spine association 7th Annual meeting, Washington D.C., 23-24. 1992.
14). Holmes R, Mooney V, Bucholz RW and Tencer A. A coralline hydroxyaptite bone graft substitute. Clin Orthop. 188:252–262. 1984.
15). Nikulin A and Ljubovic E. Der gipsstift in der experi -mentallen kochen regeneration. Acta Chir Scand. 91:17–27. 1944.
16). Peltier LF. The use of plaster of Paris to fill defects in bone. Clin Orthop. 21:1–31. 1961.
17). Peltier LF and Lillo R. The substitution of plaster of Paris rods for portions of the diaphysis of the radius in dogs. Surg Forum. 6:556–558. 1955.
18). Sigqui M, Collin P, Vitte C and Forest N. Osteoblast adherence and resorptive activity of isolated osteoclasts on calcium sulfate hemihydrate. Biomaterials. 16:1327–1332. 1995.
19). Summers BN and Eisenstein SM. Donor site pain from the ilium: A complication of lumbar spine fusion. J Bone Joint Surg. B:667–680. 1989.
20). Toth JM, An HS, Lim TH, Ran Y, et al. Evaluation of porous biphasic calcium phosphate ceramics for anterior cervical interbody fusion in a canine model. Spine. 20:2203–2250. 1955.
22). Urist MR and Dawson E. Intertransverse process fusion with the aid of chemosterilized autolyzed antigen-extract -ed allogenic (AAA) bore. Clin Orthop. 165:97–113. 1981.
23). Yamazaki Y, Oida S, Akinoto Y and Shoida S. Response of the mouse femoral muscle to an implant of a composite of bone morphogenic protein and plaster of Paris. Clin Orthop. 234:240–249. 1988.
24). Younger EM and Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma. 3:192–195. 1989.
Go to : 
 | Fig. 1-A.Postoperative roentgenogram Fig. 1-B. Postoperative 2 years. Complete union was noted in both graft sites. Fig. 1-C. On CT finding, there was bone formation on both transverse processes. |
 | Fig. 2-A.Postoperative roentgenogram Fig. 2-B. Postoeprative 28 months. Calcium sulfate site was completely resorbed and showed nonunion. Fig. 2-C,D. No bony union in CS mixed with autogenous bone graft site, but were showing bony union on autogenous bone graft site. |
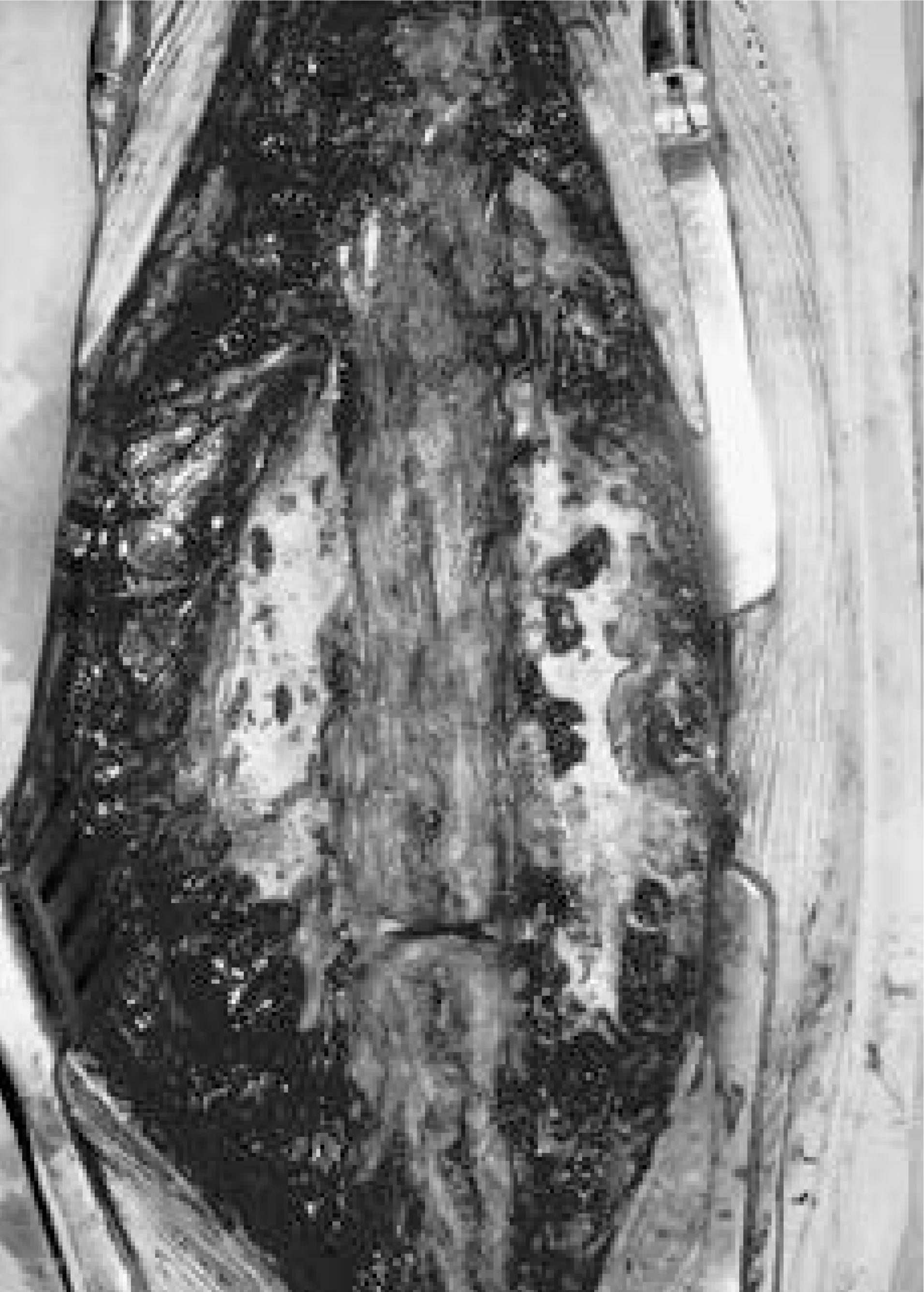 | Fig. 3.Showing multiple pores in CS mixed with AGB site(Rt) as compared with autogenous graft(Lt) on gross findings. |
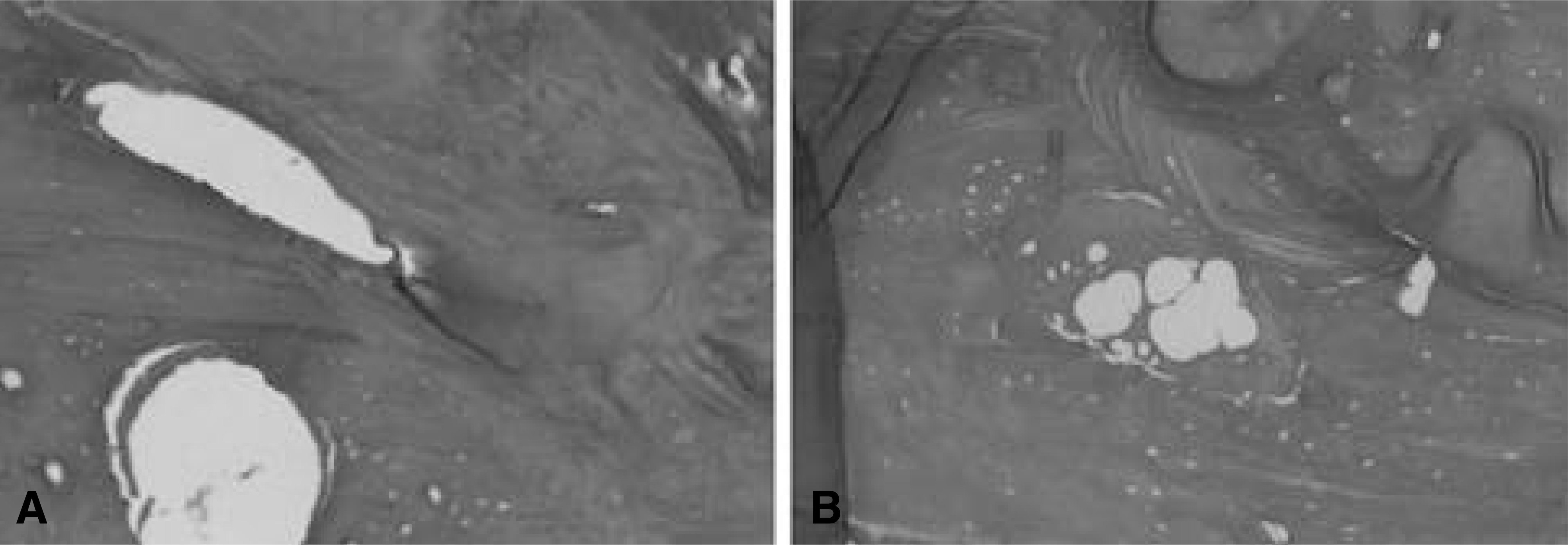 | Fig. 4.Showing bony trabeculae. But osteocytes were not visible. There was no histologic difference between both groups in matrix (× 100, H-E staining). A: AGB site, B: CS mixed with AGB site |
Table 1.
Summary of Clinical Data
∗ FBSS : Failed back surgery syndrome () : numeric number means nonunion segment Cx. : complication Assessment of fusion according to Lenke’s classification A : Indicates a big, solid trabeculated bilateral fusion mass(definitely solid) Β : Big, solid unilateral fusion mass with a small controlateral fusion mass(possibly not solid) C : Small, thin bilateral fusion mass with an apparent crack(possibly not solid) D : Bilateral resorption of the graft with an obvious bilateral pseudoarthrosis(not solid)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download