Abstract
Study Design
A retrospective analysis about related diagnostic and therapeutic factors in postoperative deep infection cases after posterior spinal instrumentation.
Objectives
A nalysis of the inherent risk factors associated with deep infection and the efficacy of management with prolonged suction drainage without removal of implants.
Summary of Literature Review
Various treatment modalities have been applied to control deep infection after spinal instrumentation. Validity of removing implants to control the infection is still controversial because it may cause loss of spinal stability.
Materials and Methods
Five cases of postoperative deep infection after posterior spinal fixation from May 1996 to May 2000 were investigated about combined general illness, features of infection, various profiles on management of the infection with surgical irrigation and debridement followed by prolonged suction drainage, and final outcomes.
Results
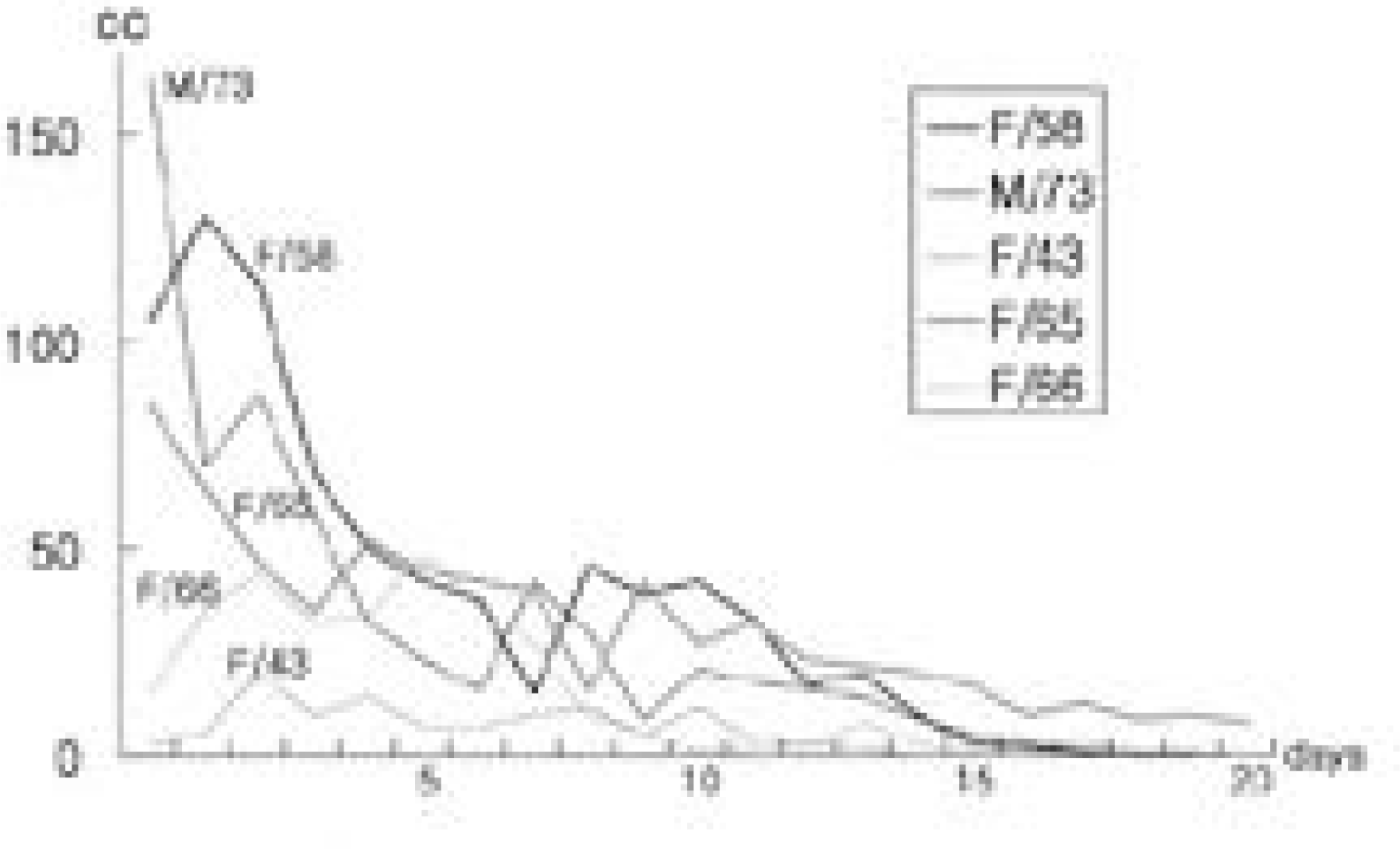
Remarkable risk factors were diabetes and obesity. Evidences of infection such as discharge from the wound, dehiscence, fever were observed since average 18.8th day postoperatively. By only one surgical procedure for each patient followed by prolonged suction drainage for mean 19.2 days and administration of IV antibiotics for average 43.6 days followed by oral antibiotics for 33.8 days, deep infections were controlled successfully without removal of implants and without any grave complications. All achieved favorable clinical results and posterolateral fusion.
Conclusion
Irrigation and debridement accompanied by prolonged suction drainage using Hemovac and administration of susceptible antibiotics seemed to be one of the effective methods in controlling deep infection after posterior instrumentation and in maintaining the postoperative stability of spine.
Go to : 
REFERENCES
1). Abbey DM, Turner DM, Warson JS, Wirt TC, Scalley RD. Treatment of postoperative wound infections following spinal fusion with instrumentation. J Spinal Dis. 8:278–283. 1995.

2). Chung YG, Ha KY. Adherence and biofilm formation of staphylococcus epidermidis and mycobacterium tuberculosis on spinal inplant. J Kor Spine Surg. 6:47–56. 1999.
3). Eysel P, Hopf Ch, Vogel I, Rompe JD. Primary sta -ble anterior instrumentation or dorsoventral spondylodesis in spondylodiscitis? Eur Spine J. 6:152–157. 1997.
4). Gristina AG, Winston-salem , Carolina N, Coster-ton JW. Bacterial adherence to biomaterials and tissue. J Bone Joint Surg. 67(A):264–273. 1985.
5). Gepstein R, Eismont FJ. Postoperative spine infections. Complications of Spine surgery. Garfin SR, editor. Balti -more: Williams and Wilkins;p. 302–322. 1989.
6). Jeong ST, Kim JY, Park YJ, Koo KH, Song HR. Treatment of wound infection using antibiotics-mixed bone cement beads as complication of spine surgery. J Kor Spine Surg. 3:225–231. 1996.
7). Keller RB, Pappas AM. Infections after spinal fusion using internal fixation instrumentation, Orthop Clin North America. 3:99–111. 1972.
8). Kim EH, Song IS. Deep wound infection after lumbar spine fusion with pedicular screw fixation. J Kor Spine Surg. 7:535–543. 2000.
9). Kim KS, Ko SH, Im CI, Kim DY. Postoperative wound infection in patients with posterior spinal instrumentations. J Kor Spine Surg. 3:77–82. 1996.
10). Kim YH. Total knee arthroplasty for tuberculosis arthritis. J Bone Joint Surg. 70(A):1322–1330. 1988.
11). Lonstein J, Winter R, Moe JH. Wound infection with Harrington instrumentation and spine fusion for scoliosis.
12). Massie JB, Heller JG, Abitbol JJ, McPherson D, Garfin SR. Postoperative posterior spinal wound infection. Clin Orthop. 284:99–108. 1992.
13). McAfee PC, Bohlman HH. Complications following Harrington instrumentation for fractures of the thoracolumbar spine. J Bone Joint Surg. 67:672–685. 1985.

14). Park WW, Park YS, Cheon SJ, Jung JY. Posterior lumbar interbody fusion in the pyogenic discitis. J Kor Spine Surg. 8:39–45. 2001.

15). Picada R, Winter RB, Lonstein JE, Denis F, Pinto MR. Postoperative deep wound infection in adults after posterior lumbosacral spine fusion with instrumentation. incidence and management. J Spinal Dis. 13:42–45. 2000.

16). Rah JH, Ahn JI, Park HJ. A clinical study of postoperative infection in posterior spinal surgery with pedicle screw system. J Kor Orthop Asso. 29:994–1001. 1994.
17). Richards BS. Delayed infections following posterior spinal instrumentation for the treatment of idiopathic scoliosis. J Bone Joint Surg. 77:524–529. 1995.

18). Shim DM, Kim TK, Song HH, Shim YS, Lee SH, Song JH. Quantitation of C-reactive protein levels and erythrocyte sedimentation rate after spinal surgery. J Kor Spine Surg. 5:33–39. 1998.
19). Smith AJ, Bloser SI. Infections and inflammatory processes of the spine. Radiol Clin North Am. 29:809–823. 1991.
20). Thelander U, Larsson S. Quantitation of C-reactive protein levels and erythrocyte sedimentation rate after spinal surgery. Spine. 17:400–403. 1991.

21). Viola RW, King HA, Adler SM, Wilson CB. Delayed infection after elective spinal instrumentation and fusion. A retrospective analysis of eight cases. Spine. 22:2444–2450. 1997.
22). Weinstein MA, McCabe JP, Cammisa FP. P ostope rative spinal wound infection. A review of 2391 consecutive index procedures. J Spinal Dis. 13(5):422–426. 2000.
23). Weiss LE, Vaccaro AR, Scuderi G, McGuire M, Garfin SR. Pseudarthrosis after postoperative wound infection in the lumbar spine. J Spinal Dis. 10:482–487. 1997.

24). Wright DL. Septic complication of neurosurgical spinal procedure. Spiringfield: IL. Chales C Thomas;1970.
Go to : 
Figures and Tables%
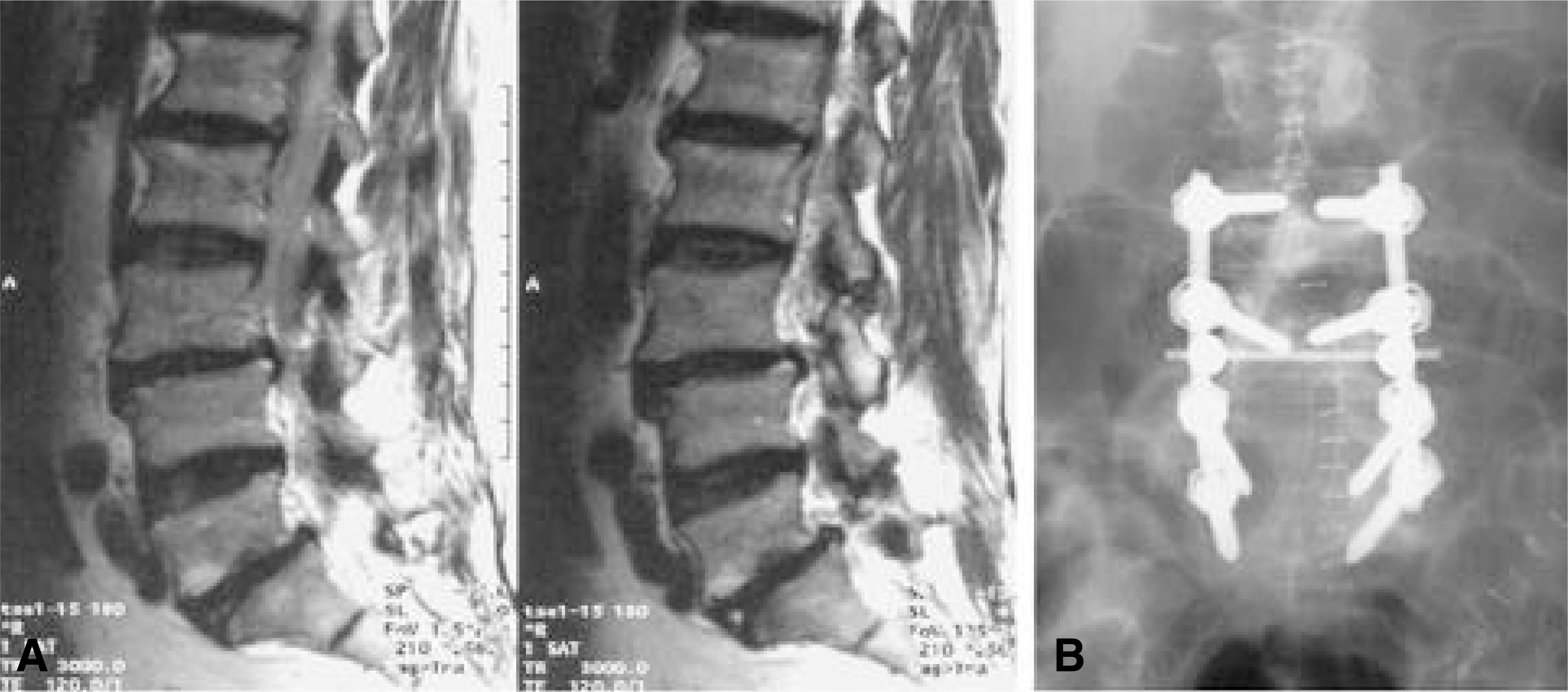 | Fig. 2-A.T2 sagittal MR image shows spondylolisthesis of L3-4, L5-S1 and multilevel stenosis in a 58 year-old lady with Diabetes and obesity. Fig. 2-B. Posterior decompression and posterolateral fusion with transpedicular screw system including transverse fixation were performed. |
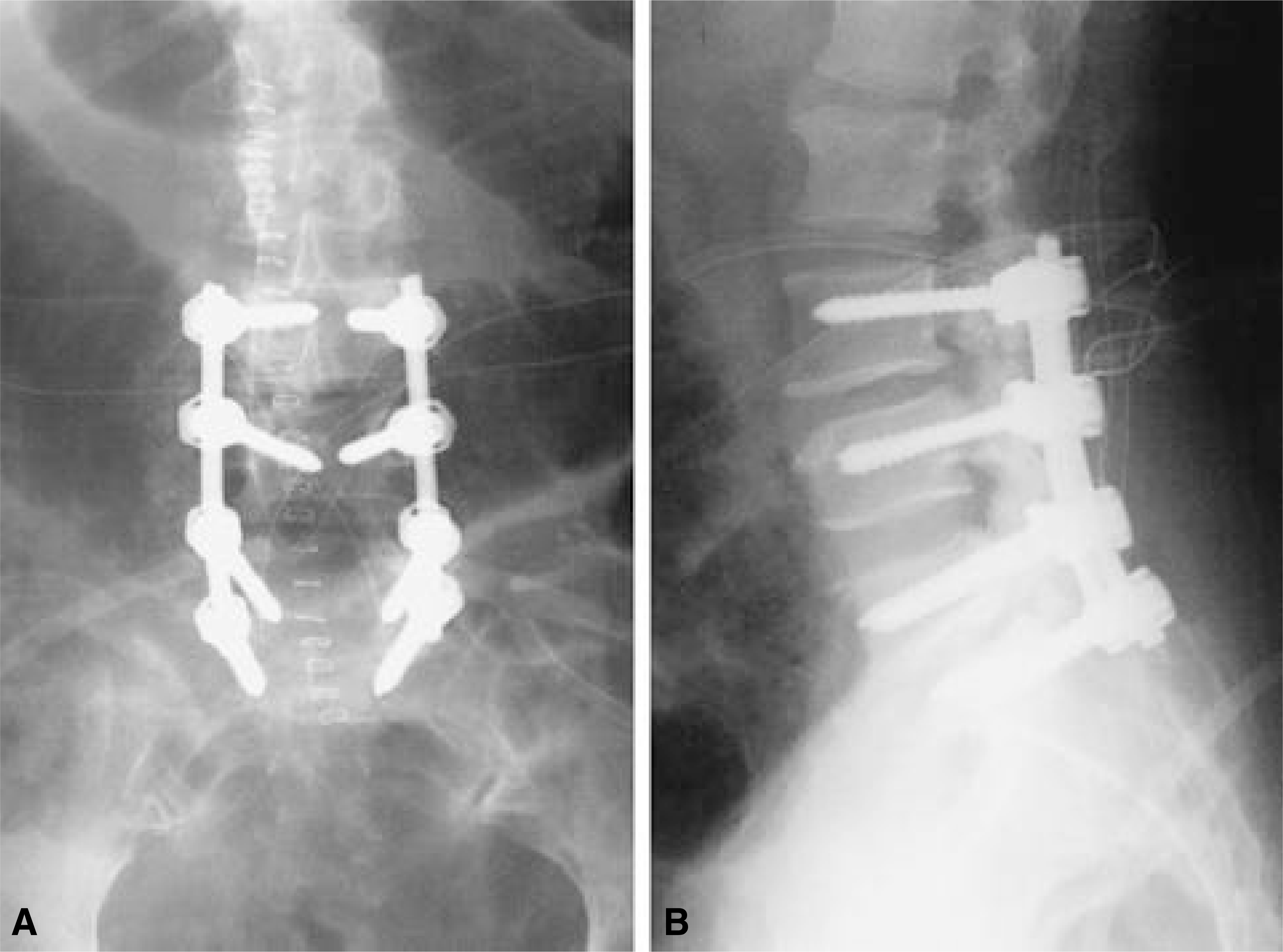 | Fig. 4-A.On 14th day, the wound was reopened for thorough irrigation and debridement. TLC bar was removed to minimize dead space. Two pairs of Hemovac were inserted for drainage. Fig. 4-B. Postoperative lateral X-ray. |
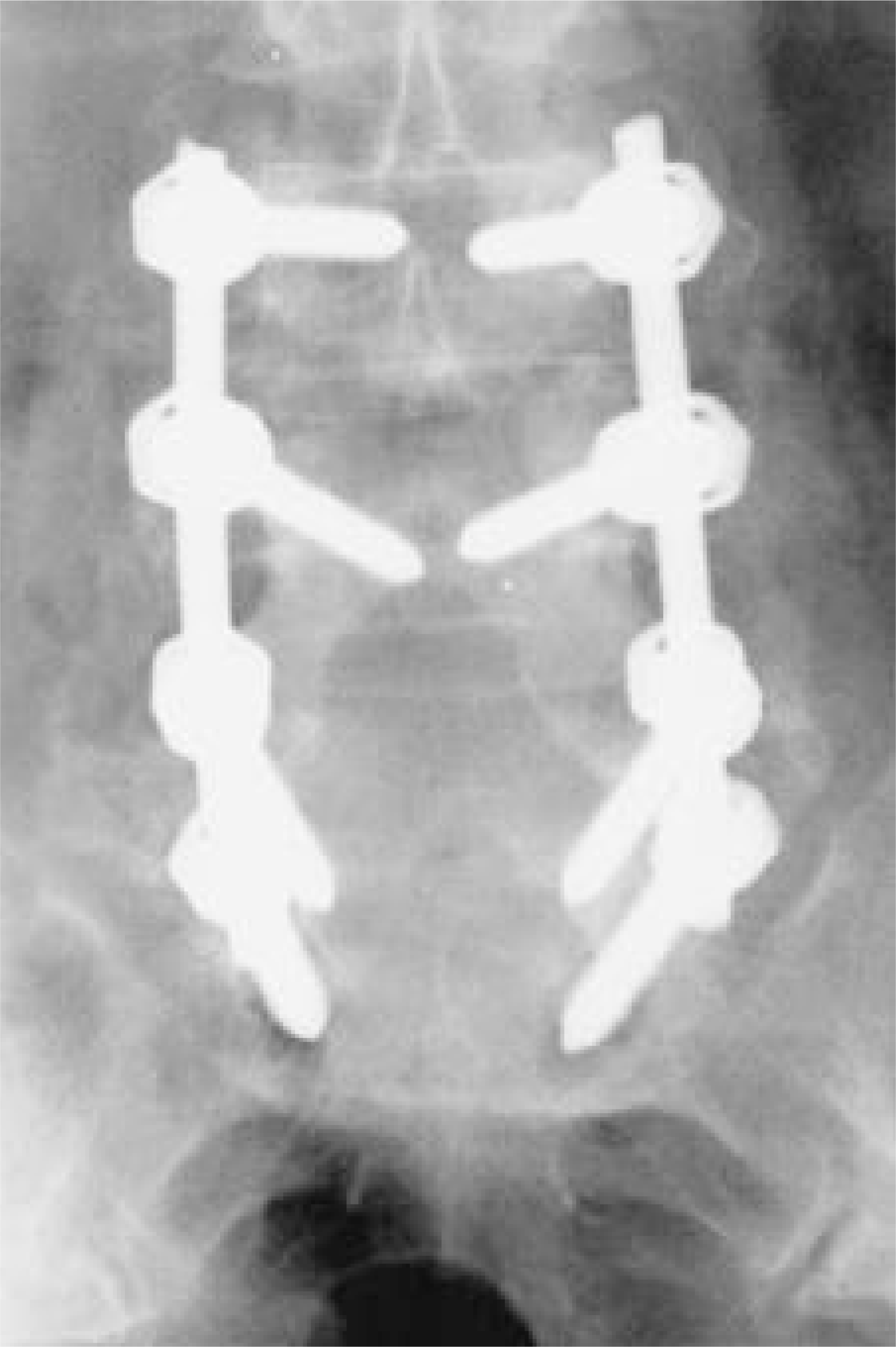 | Fig. 5.Anteroposterior X-ray at postoperative 6 month shows bridging of posterolateral fusion mass. |
Table 1.
Profiles of Deep Infection after Posterior Spinal Instrumentation
| Case | Sex/Age | Associated Problems | Initial Diagnosis | Fusion Level | Onset of Infection | Infection Sign Fever | ESR/CRP |
|---|---|---|---|---|---|---|---|
| DM Obesity | |||||||
| 1 | F/58 | Hypertension | Spinal stenosis | L3-S1 | PO 8 days | Wd discharge | 103 / 20.4 |
| Hypertension | |||||||
| 2 | M/73 | DM COPD | Spinal stenosis | L4-S1 | PO 18 days | Wd discharge | 25 / 5.4 |
| Wd dehiscence | |||||||
| DM Obesity | Wd dehiscence | ||||||
| 3 | F/43 | Spinal stenosis | L4-S1 | PO 16 days | Wd discharge | 38 / 4.8 | |
| Hypertension | |||||||
| Wd swelling | |||||||
| 4 | F/65 | DM | Spinal stenosis | L2-S1 | PO 36 days | & pain | 72 / 8.1 |
| Malnutrition | Spinal stenosis | T12-L2, | & pain Wd discharge | ||||
| 5∗ | F/66 | Malnutrition | Spinal stenosis | T12-L2, | PO 16 days | Wd discharge | 49 / 21.2 |
| Anemia | L1 compression Fx. | L4-5 | Fever |
Table 2.
Management of Infection and Results




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download