Abstract
Purpose
The purpose of the present study was to compare the osteoconduction in porous bodies made of various compositions of calcium phosphate compounds and other porous artificial bones.
Materials and Methods
Single- level posterolateral spinal fusions were performed on ninety rabbits. The animals were divided into nine groups by graft materials: autograft (positive control), implantation of HA, TCP, CPP, HA /TCP composite, TCP/CPP composite, Lubboc Ⓡ and Calcium sulfate pellet (CSP), nograft after decortication (negative control). Serial radiography, serum calcium and phosphorus levels were checked. All animals were sacrificed 12 weeks after surgery and the fusion masses were compared by manual palpation, uniaxial tensile strength measurement and histological evaluation.
Results
A utografted and CPP implanted groups showed significantly higher fusion ratio than TCP, TCP/CPP composite, and nograft groups. Meanwhile, HA and HA /TCP groups showed no significant difference with other groups in fusion ratio. From the radiological examination, TCP and CPP groups seemed to show more rapid absorption of implant than HA group. The mean values of tensile strength of autografted and CPP group were significantly larger than those of TCP, TCP/CPP composite, and nograft groups. The result of direct inspection and microscopic examination showed the TCP- contained implants lost their porous structure, whereas the other implants did not. On the light microscopy, both HA and CPP groups showed more abundant new bone growth into the pores than TCP- contained groups, but the pore size of CPP became larger than that of the HA, which manifested more rapid absorption of CPP in the living body.
Go to : 
REFERENCES
1). Boden SD, Schimandle H, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine. 20:412–20. 1995.
2). Bucholz RW, Carlton A, Holmes RE. Hydroxyapatite and tricalcium phosphate bone graft substitute. Orthop Clin North Am. 18:323–334. 1987.
3). Chung SS, Hong KS, Youn HJ, Chang BS, Yeom JS, Lee CK, Park YK, Ryu HS, Park KW. Effects of Pore Size on Osteoconduction at the Porous Hydroxyapatite. J Korean Orthop Assoc. 33:158–167. 1998.

4). De Aza PN, Guitian F, De Aza S. Bioeutectic: a new ceramic material for human bone replacement. Biomaterials. 18(19):1285–1291. 1997.

5). Freidlaender GE. Current concepts review: Bone grafts. J Bone Joint Surg. 69-A:786–790. 1987.
7). Holmes RE, Bucholz RW, Monney V. Porous hydroxyapatite as a bonegraft substitute in metaphyseal defects. J Bone Joint Surg. 68-A:904–911. 1986.
8). Ishihara K, Arai H, Nakabayashi N, Morita S, Fu-ruya K. Adhesive bone cement containing hydroxyapatite particle as bone compatible filler. J Biomed Mater Res. 26:937–945. 1992.

9). Ishikawa K, Ducheyne P, Radin S. Determination of the Ca/P ratio in calcium-deficient hydroxyapatite using X-ray diffraction analysis. J mater Sci Med. 4:165–168. 1993.

10). Klein CP, Driessen AA, de Groot K, van den Hooff A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J Biomed Mat Res. 17:769–784. 1983.

11). Lee CK, Cho KH, Suh H, Ahn SJ. A radiological and histological study of carbonate apatite collagen composite and hydroxyapatite implanted in bone defects of the rabbit tibiae. J Korean Orthop Assoc. 30:1109–1118. 1995.

12). Tsuruga E, Takita H, Itoh H, Wakisaka Y, Kuboki Y. Pore size of porous hydroxyapatite as the cell substra -tum controls BMP-induced osteogenesis. J Biochem. 121:317–324. 1997.
13). Yamamura K, Iwata H, Yotsuyanagi T. Synthesis of antibiotic-loaded hydroxyapatite beads and in vitro drug release testing. J Biomed Mater Res. 26:1053–1064. 1992.
Go to : 
Figures and Tables%
 | Fig. 1.Uniaxial tensile testing to failure was performed. The motion segments were pulled apart using screws that were placed through the vertebral bodies and connected to crosshead of the machine by link chain as shown. |
 | Fig. 2.Serial radiographs of rabbits in autografted and Lubboc® implanted groups. Autogenous corticocancellous bone was trans-formed to homogeneous fusion masses at 12 weeks. Lubboc® granules showed lower radiopacity than calcium phosphate grafts initially and seemed to form homogeneous fusion masses at 6 weeks. |
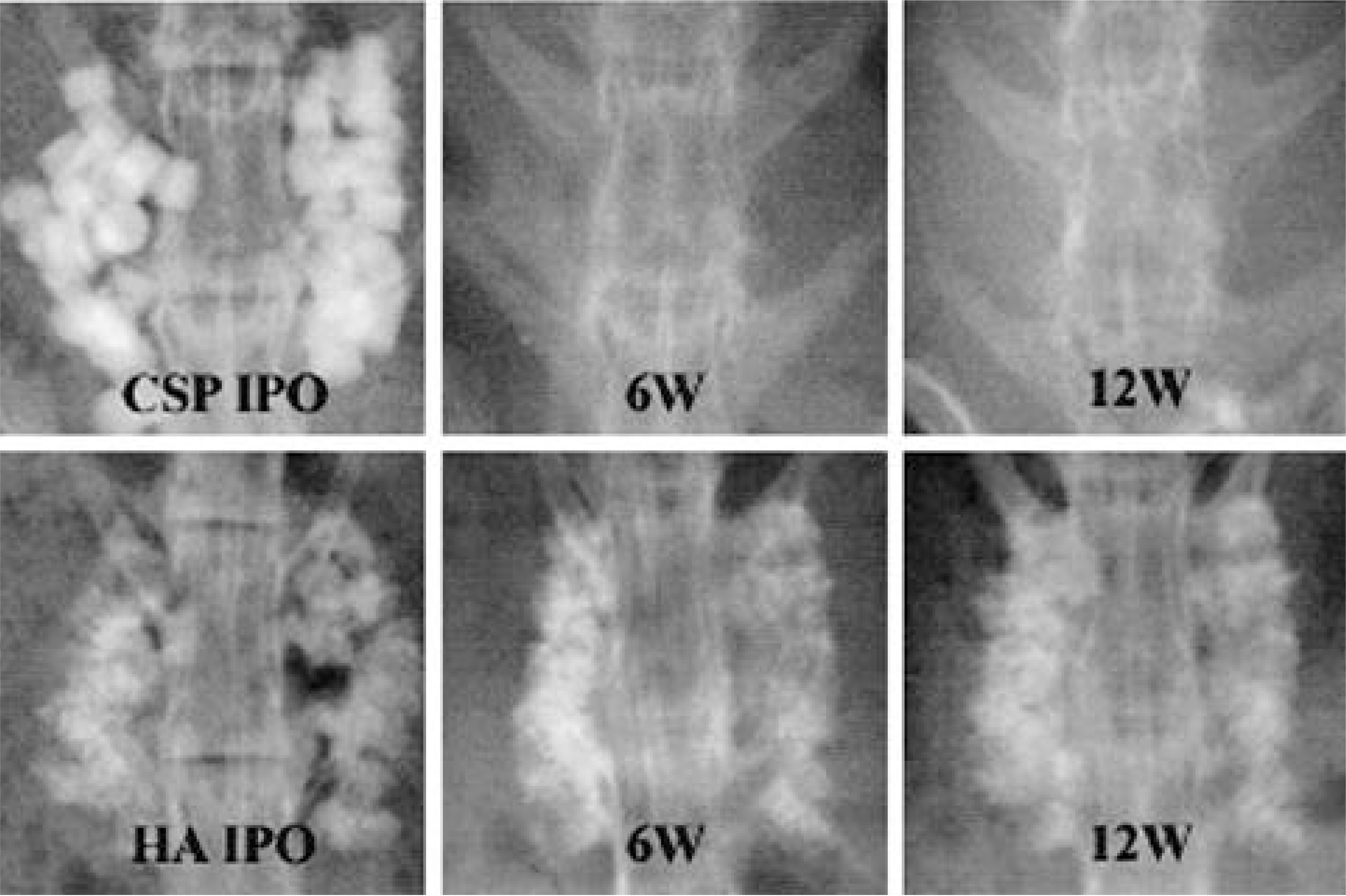 | Fig. 3.Serial radiographs of rabbits in CSP and HA implanted groups. Calcium sulfate pellets were dissolved completely at 6 weeks and no evidence of new bone formation was visible between upper and lower transverse processes. Hydroxyapatite granules showed marginal blurring at 6 weeks, however, the radio-opacities of centers of granules was sustained up to 12 weeks. |
 | Fig. 4.Serial radiographs of rabbits in TCP and CPP implanted groups. Tricalcium phosphate granules lost their discrete margins at 4 weeks and formed diffuse radio-opaque shadows between transverse processes. Calcium pyrophosphate grafts showed more rapid dissolution and formed more homogeneous fusion masses than HA. |
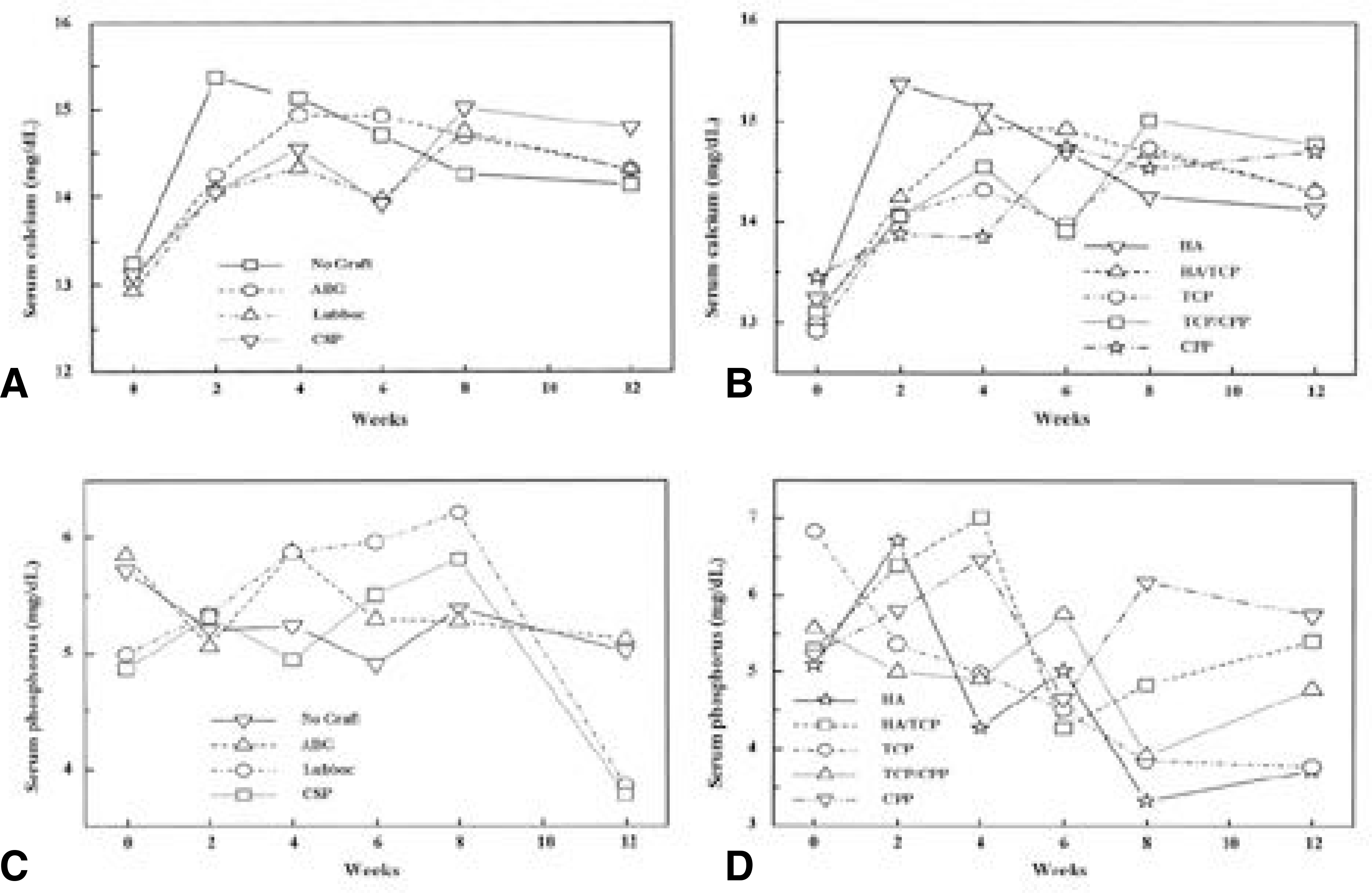 | Fig. 5.In all the groups, serum calcium was increased slightly after operation, however, there was no significant difference between each group (A, B). Serum phosphorus level was highly variable according to time and cases (C, D). |
 | Fig. 6.Photographs of harvested specimens. Autograft (A), HA (E), and CPP (H) grafts formed rigid fusion masses between transverse processes. Meanwhile, the cases in no-graft (B) and CSP (D) groups showed new bone was not conducted. Lubboc® (C), TCP (F), and TCP/CPP (G) grafts showed collapsed porous structure of implant and relatively scanty fusion masses. |
 | Fig. 7.Histological findings of fusion masses (H&E stain, × 12.5; A, decalcified, B-F, undecalcified). Autograft (A) was trans-formed to mature bone. Lubboc® granules conducted in-growth of new bone and considerable amount of fibrous tissue. Calcium sulfate pellets (C) were completely dissolved and remained defect was filled with fibrous tissue. TCP implant (E) lost its porous structure and new bone in-growth ceased at collpased pore. HA (D) and CPP (F) graft maintained porous structures and lamellated mature bone grew into the pores. The walls of pores in CPP graft were thinner than those in HA, which suggested more rapid resorption of CPP. |
Table 1.
Animal groups according to graft compositions
Table 2.
Comparison of Fusion Ratio and Mean Tensile Strength
| Group | Fusion ratio on manual palpation | Mean tensile strength (N) |
|---|---|---|
| Autograft | 7/7∗∗ | 138±9 |
| No Graft | 0/9∗∗ | 0∗∗ |
| Lubboc® | 6/7 | 102±43∗∗ |
| CSP | 0/10∗∗ | 0∗∗ |
| HA | 8/10 | 189±46* |
| HA/ß-TCP | 6/10 | 128±54∗∗ |
| ß-TCP | 3/8∗∗ | 120±10∗∗ |
| ß-TCP/ß-CPP | 5/10∗∗ | 140±53 |
| ß-CPP | 7/7* | 191±56* |
| CSP: Calcium Sulfate Pellet | ||
| HA: Hydroxyapatite, TCP: Tricalcium Phosphate, | ||
| CPP: Calcium Pyrophosphate | ||




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download