Abstract
Background
Genetic modification of cells through gene transfer gains popularity as a sophisticated delivery system in the management of musculoskeletal disease. A nabolic growth factors like IGF- 1BMP- 2 OP- 1, and TGF- ß1 were candidate for therapeutic purposus for regenerating matrix of intervertebral disc. TGF- ß1, OP- 1 and BMP- 2 share same pathway i.e. Smad while IGF- 1 utilize P13K pathway. A ccordingly it is logical to use two cytokine encoding genes which using different signal transduction pathway to upregulate matrix synthesis.
Purpose
To elucidate the anabolic effect of the combination gene transfer(IGF- 1 and TGF- ß1 encoding gene) to human disc cells, cultured in alginate beads.
Materials and Methods
Lumbar and cervical intervertebral disc tissue was obtained from surgical disc procedure from fifteen patients. A fter isolation and culture of the cells, cultures were transduced with first, A denovirus- TGFß1construct(A d/TGF- ß1) and A d/IGF- 1 respectively, second, with combination of two viral constructs(A d/TGF- ß1+A d/IGF- 1). Cultures treated with saline and A d/luciferase served as control. Then cultures were incorporated into alginate beads. Exogenous TGF- ß1(2 ng/ml) and IGF- 1(100 ng/ml) were administered also. Newly synthesized proteoglycan was assessed using S35 incorporation using chromatography on Sephadex G- 25 in PD- 10 column
Results
In cultures transduced with single therapeutic gene construct, there were statistically significant 2.9 fold(A d/TGF- ß1) and 1.8 fold(A d/IGF- 1) increase in newly synthesized proteoglycan comparing control(p<0.05). In culture transduced with double combination of therapeutic gene construct, there were 3.9 fold(A d/TGF- ß1+A d/IGF- 1) increase in newly synthesized proteoglycan comparing control(p<0.05). Culture treated with TGF- ß1(2ng/ml) showed 3.9 fold increase and IGF- 1(100 ng/ml) 2.9 fold increase, TGF- ß1+IGF- 1 4.2 fold increase in proteoglycan synthesis compared to control.
Go to : 
REFERENCES
1). Borenstein D. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr Opin Rheumatol. 4:226–32. 1992.

2). Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 20:1307–1314. 1995.

3). Butler D, Trafimow JH, Andersson GB, McNeill TW, Huckman MS. Discs degenerate before facets. Spine. 15:111–113. 1990.

4). Chelberg MK, Banks GM, Geiger DF, Oegema TR. Identification of heterogenous cell populations in normal human intervertebral disc. J Anat. 186:43–53. 1995.
5). Crabb ID, O'Keefe RJ, Edward Puzas J, Rosier RN. Synergistic effect of transforming growth factor beta and fibroblast growth factor on DNA synthesis in chicken growth plate chondrocytes. J Bone Miner Res. 5:1105–1112. 1990.
6). Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J Bone Joint Surg. 77-A:1103–1114. 1995.

7). Evans CH, Robbins PD. Genetically augmented tissue engineering of the musculoskeletal system. Clin Orthop. 367:S410–8. 1999.

8). Evans CH, Robbins PD. Potential treatment of osteoarthritis by gene therapy. Rheum Dis Clin North Am. 25:333–44. 1999.

9). Eyre D, Benya P, Buckwalter J, Frymoyer JW, Gordon SL. Intervertebral disc: Part B. Basic science perspective. New Perspectives in Low Back Pain. Park Ridge, IL: American Academy of Orthopaedic Surgeon;p. 147–207. 1989.
10). Gruber HE, Fisher EC, Desani B, Stasky AA, Hoelscher G, Hanley EN. Human intervertebral disc cells from the annulus: three dimensional culture in agarose or alginate and responsiveness to TGF-b1. Exp Cell Res. 235:13–21. 1997.
12). Itoh S, Itoh F, Goumans M-J, Dijke PT. Signalling of transforming growth factor-ß family members through Smad protein. Eur J Biochem. 267:6954–6967. 2000.
13). Lipson SJ, Muir H. Proteoglycans in experimental intervertebral disc degeneration. Spine. 6:194–210. 1981.

14). Maldonado BA, Oegema TR. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 10:677–690. 1992.

15). Melrose J, Ghosh P, Taylor TK, Hall A, Osti OL, Ver-non-Roberts B, Fraser RD. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibosus. J Orthop Res. 10:665–676. 1992.
16). Moon SH, Kang JD, Nishida K, Gilbertson LG, Niyibizi C, Smith PN, Knaub MA, Robbins PD, Evans CH. Human cervical intervertebral disc cells are susceptible to adenovirus-mediated gene therapy. Proceedings of Cervical Spine Research Society, p150-153. 1999.
17). Moon SH, Nishida K, Kang JD, Gilbertson LG, Muz-zonigro TS, Knaub MA, Robbins PD, Evans CH. Human intervertebral disc cells are genetically modifiable in-vitro by adenovirus-meidated gene transfer: Implications for the clinical management of intervertebral disc dis -order. Spine. 25:2573–2579. 2000.
18). Nishida K, Kang JD, Gilbertson LG, Moon S-H, Suh J-K, Vogt MT, Robbins PD, Evans CH. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: An in vivo study of adenovirus-mediated transfer of the human transforming growth factor ß 1 encoding gene. Spine. 24:2419–2425. 1999.
19). Nishida K, Kang JD, Suh J-K, Robbins PD, Evans CH, Gilbertson LG. Adenovirus-mediated gene transfer to nucleus pulposus cells: Implication for the treatment of intervertebral disc degeneration. Spine. 23:2437–2443. 1998.
20). Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, Tsuji H. Atocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insuline-like growth factors-1 on proteoglycan synthesis in bovine intervertebral discs. J Orhtop Res. 14:690–699. 1996.
21). Reinecke JA, Wehling P, Robbins P, Evans CH, Sager M, Schulze-Allen G, Koch H. In vitro transfer of genes in spinal tissue. Zeitschrift fur Orthopadie und Ihre Grenzgebiete. 135:412–416. 1997.
22). Takegami K, Matuda K, Kumano F, An H, Chiba K, Pankaj D, Sampath TK, Thonar EJ-MA. O steo-genic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chondroitinase ABC-induced chemonucleolysis. 45th Annual Meeting Trans Orthop Res Soc, 201. 1999.
23). Thompson JP, Oegema TR, Bradford DS. Stimulation of mature canine intervertebral disc by growth facors. Spine. 16:253–260. 1991.
24). Wehling P, Schulitz KP, Robbins PD, Evans CH, Reinecke JA. Transfer of genes to chondrocytic cells of the lumbar spine. Proposal for a treatment strategy of spinal disorders by local gene therapy. Spine. 22:1092–1097. 1997.
Go to : 
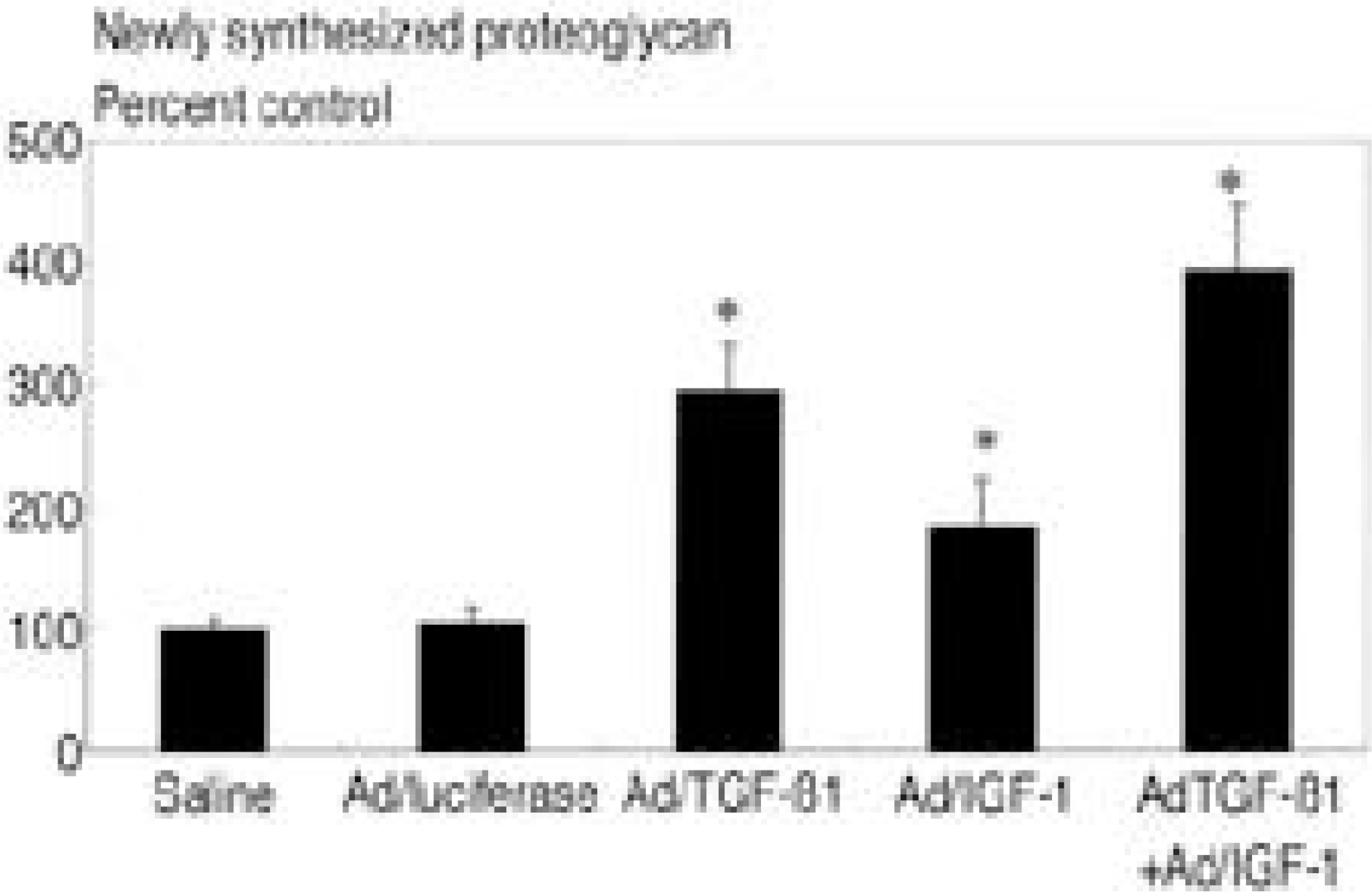 | Fig. 1.Newly synthesized proteoglycan in three dimensional culture of human intervertebral disc cells presented as percent control. Adenovirus-TGF-beta1 construct(Ad/ TGF-ß1) with an MOI of 75 showed 2.9 fold increase, adenovirus-IGF-1 construct(Ad/IGF-1) with an MOI of 75 showed 1.8 fold increase, Ad/TGF-ß1+AdIGF-1 with an MOI of 75 showed 3.9 fold increase in newly synthesized proteoglycan compared to saline and viral control. *:p<0.05 |
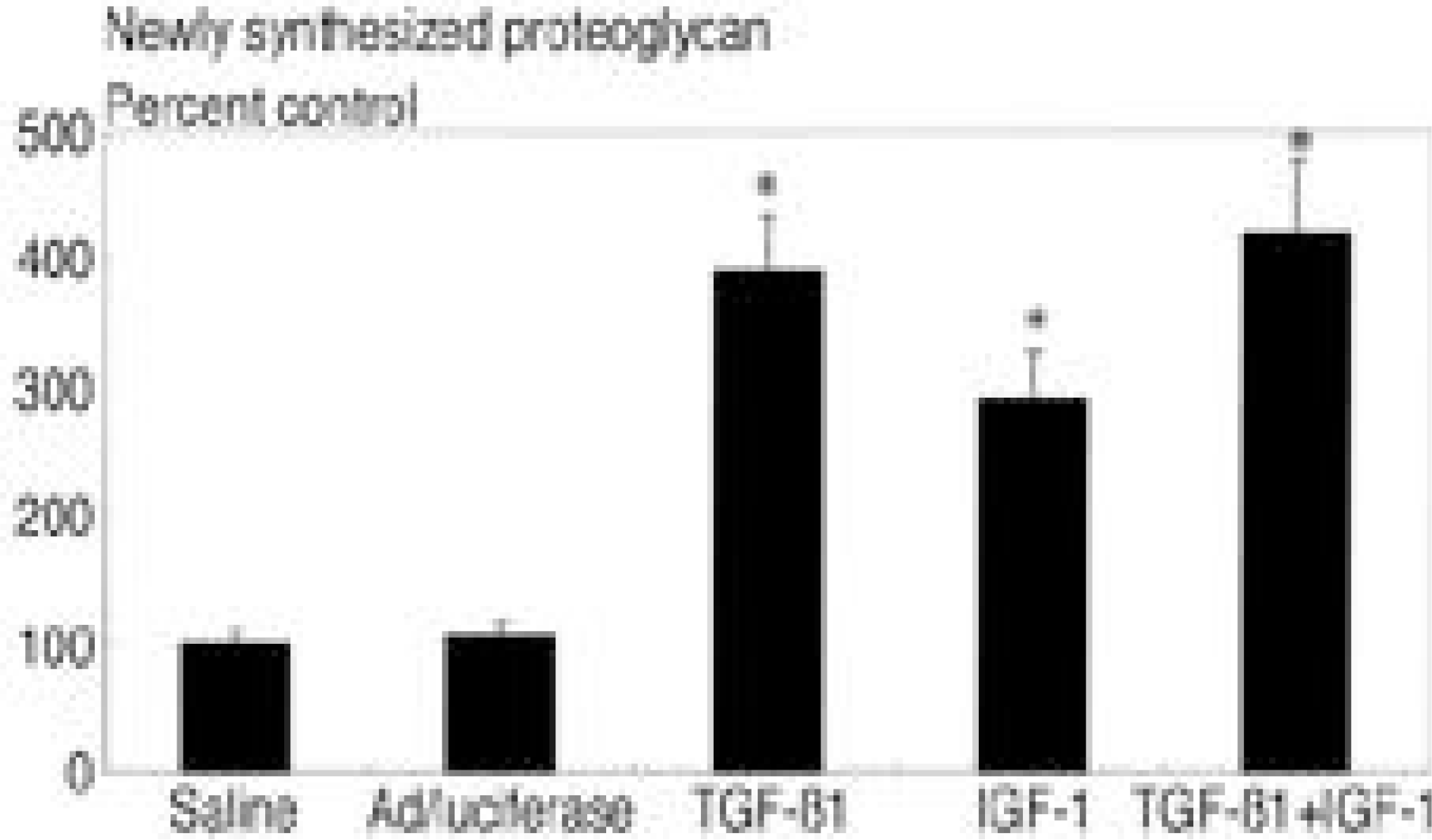 | Fig. 2.Newly synthesized proteoglycan in three dimensional culture of human intervertebral disc cells presented as percent control. TGF-ß1(2 ng/ml) showed 3.9 fold increase, IGF-1(100 ng/ml) showed 2.9 fold increase, and TGF-ß1(2 ng/ml) + IGF-1(100 ng/ml) showed 4.2 fold increase in newly synthesized proteoglycan compared to saline and viral control. *:p<0.05 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download