Abstract
Study Design
In vitro experiment to determine the matrix synthesis of human intervertebral disc (IVD) cell to adenovirus-mediated therapeutic gene transfer.
Objectives
To elucidate proteoglycan and collagen synthesis of human IVD cells in vitro to adenovirus-mediated transfer of cDNA of transforming growth factorbeta 1(TGF- β1).
Summary of Literature Review
Sophisticated method to delivery of growth factors, in continuous manner, is the genetic modi-fication of disc cells through gene transfer. Confirming susceptibility of human IVD cell to adenovirus, anabolic response of human IVD cells to therapeutic gene transfer should be next step.
Materials and Methods
IVD tissue was obtained from fourteen patients with grade III, IV degeneration. Isolation and culture of disc cells were performed. Disc cells were treated with either A d/TGF- β1exogenous TGF- β1. Control cultures were treated with either saline or A d/luciferase. Newly synthesized proteoglycans were assessed by 35S- sulfate incorporation using chro-matography on Sepadex G- 25 in PD- 10 columns. Uptake of 3 H proline was used to measure synthesis of collagen and noncollagen protein.
Results
Culture treated with A d/TGF- β1 showed 3 fold increase in proteoglycan synthesis (p<0.05), culture with exogenous TGF - β1 failed to demonstrate increase in proteoglycan synthesis. In collagen and noncollagen synthesis, cultures with A d/TGF- β1 and exogenous TGF- β1 showed similar 3.7 fold increase in collagen and 2.7 fold increase in noncollagen synthesis comparing control (p<0.05).
Go to : 
REFERENCES
1). Anderson JAD. Back pain and occupation. The lumbar Spine and Back Pain. Third Edition. MIV Jayson, editor. London: Churchill Livingstone;1987. p. 2–36.
2). Aydelotte MB, Greenhill RR and Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connect Tiss Res. 18:223–234. 1988.

3). Borenstein D. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr Opin Rheuma -tol. 4:226–232. 1992.

4). Brown GL, Curtsinger LJ, White M, et al. Acceleration of tensile strength of incisions treated with EGF and TGF-β1. Ann. Surg. 208:788–794. 1988.
5). Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 20:1307–1314. 1995.

6). Butler D, Trafimow JH, Andersson GB, et al. Di scs degenerate before facets. Spine. 15:111–113. 1990.
7). Chelberg MK, Banks GM, Geiger DF, et al. Identification of heterogenous cell populations in normal human intervertebral disc. J Anat. 186:43–53. 1995.
8). Chiba K, Andersson GBJ, Masuda K, et al. Met abolism of the extracellular matrix formed by intervertebral disc cells cultured in alginate. Spine. 22:2885–2893. 1997.
9). Evans CH and Robbins PD. Possible orthopaedic appli - cations of gene therapy. J Bone Joint Surg(Am). 77:1103–1113. 1995.
10). Eyre D, Benya P, Buckwalter J, et al. Intervertebral disc: Part B. Basic science perspective. New Perspectives in Low Back Pain. Park Ridge, IL: American Academy of Orthopaedic Surgeons;p. 147–207. 1989.
11). Graham FL and Eb Ajvd. A new technique for assay of infectivity of human adenovirus 5 DNA. Virol. 52:456–467. 1973.
12). Gruber HE, Fisher EC, Desani B, et al. Human intervertebral disc cells from the annulus: three dimensional culture in agarose or alginate and responsiveness to TGF-b1. Exp Cell Res. 235:13–21. 1997.
13). Guo J, Jourdian GW, McCallum DK. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Conn Tiss Res. 19:277–97. 1989.

14). Hauselmann HJ, Fernandes RJ, Mok SS, et al. Pheno -typic stability of bovine articular chondrocytes after long-term culture in alginate beads. J Cell Sci. 107:17–27. 1994.
15). Hutton WC, Toribatake Y, Elmer WA, et al. The effect of compressive force applied to the intervertebral disc in vivo. Spine. 23:2524–2537. 1998.

16). Lipson SJ and Muir H. Proteoglycans in experimental intervertebral disc degeneration. Spine. 6:194–210. 1981.

17). Maldonado BA and Oegema TR. Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 10:677–690. 1992.
18). Mittereder N, March KL and Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 70:7498–7509. 1996.

19). Moon S-H, Gilbertson LG, Nishida K, et al. H uman intervertebral disc cells are genetically modifiable by ade -novirus-mediated gene transfer: Implications for the clinical management of intervertebral disc disorder. Spine. 25:2573–2579. 2000.
20). Moon S-H, Kang JD, Nishida K, et al. Human cervical intervertebral disc cells are susceptible to adenovirus-mediated gene therapy. Proceedings of Cervical Spine Research Society. 1999.
21). Nishida K, Kang JD, Gilbertson LG, et al. Modulation of biologic activity of the rabbit intervertebral disc by gene therapy: An in vivo study of adenovirus-mediated transfer of the human transforming growth factors 1 encoding gene. Spine. 24:2419–2425. 1999.
22). Nishida K, Kang JD, Suh J-K, et al. Adenovirus-mediat -ed gene transfer to nucleus pulposus cells: Implication for the treatment of intervertebral disc degeneration. Spine. 23:2437–2443. 1998.
23). Osada R, Oshima H, Ishihara H, et al. Autocrine/parac-rine mechanism of insulin-like growth factor-1 secretion, and the effect of insuline-like growth factors-1 on proteoglycan synthesis in bovine intervertebral discs. J Orhtop Res. 14:690–699. 1996.
24). Price J, Turner D, Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci. 84:156–160. 1987.

25). Robbins PD and Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 80:35–47. 1998.
26). Sanes JR, Rubenstein JLR, Nicholas JF. Use of a recom -binant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 5:5133–5142. 1986.
27). Siegel JA, Lonner BS, Grande DA, James T. The effect of transforming growth factor-beta on intervertebral disc tissue. Proceedings of ISSLS, 213A, Kona Hawaii. 1999.
28). Sprugel KH, McPherson JM, Clowes AW, et al. Effect of growth factors in vivo. Am J Pathol. 129:601–613. 1987.
29). Takemi K, Kumano F, An H, et al. Osteogenic protein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chon -droitinase ABC-induced chemonucleolysis. Tranc Orthop Res Soc. 201:1999.
30). Thompson JP, Oegema TR Jr and Bradford DS. Stim -ulation of mature cannine intervertebral disc by growth factors. Spine. 16:253–260. 1991.
31). Yeh P and Perricaudet M. Advances in adenoviral vectors: from genetic engineering to their biology. FASEB Journal. 11:615–623. 1997.
Go to : 
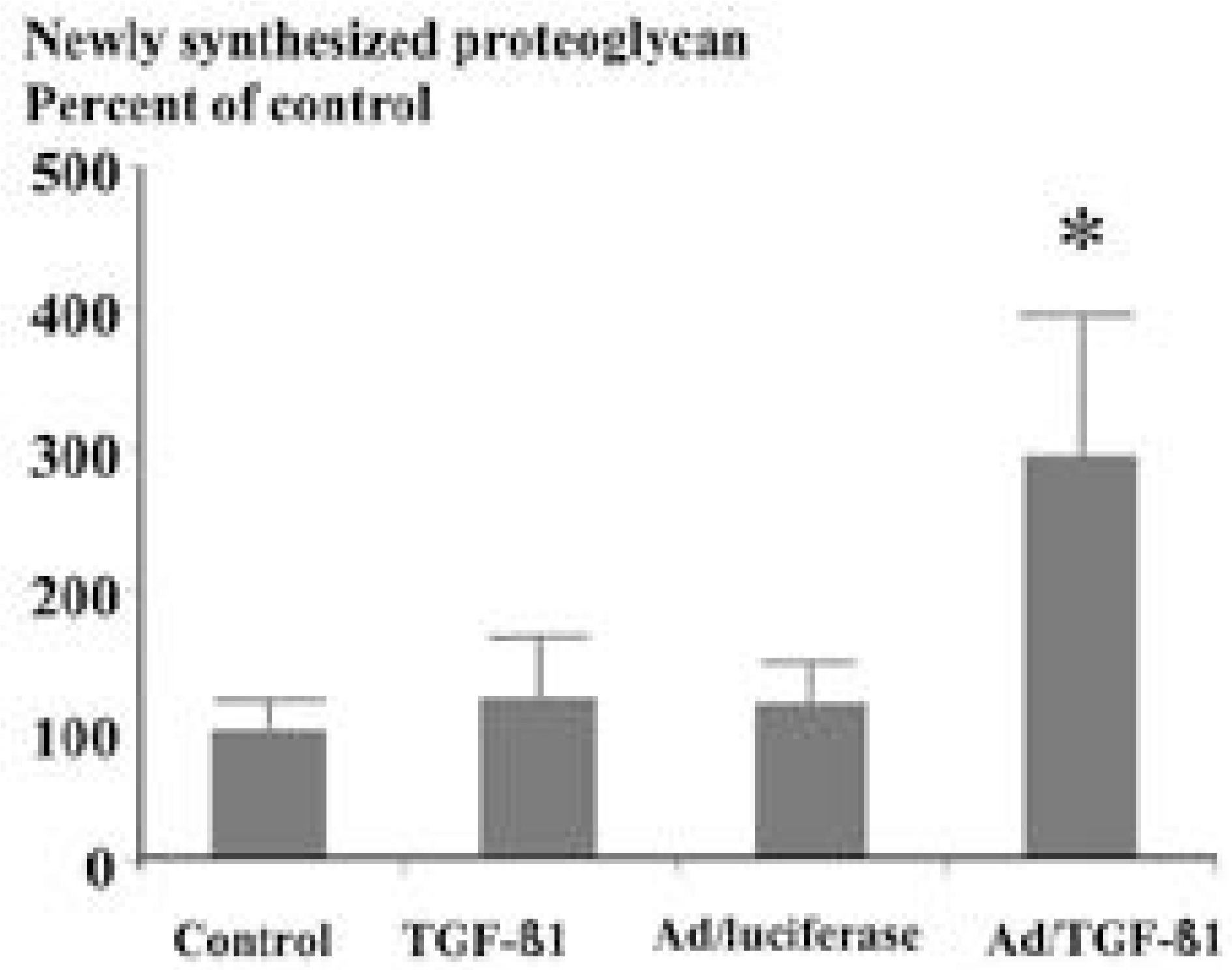 | Fig. 1.Content of newly synthesized proteoglycan as assayed by incorporation of 35S-sulfate. Human intervertebral disc cells transduced by adenovirus-TGF β1 construct(150 MOI) showed 3 fold increase in newly synthesized proteoglycan compared to those treated with normal saline∗(p<0.05), while culture treated by TGF-β1(2 ng/ml) showed no increase in newly synthesized proteoglycan compared to those treated with normal saline(p=0.07). |
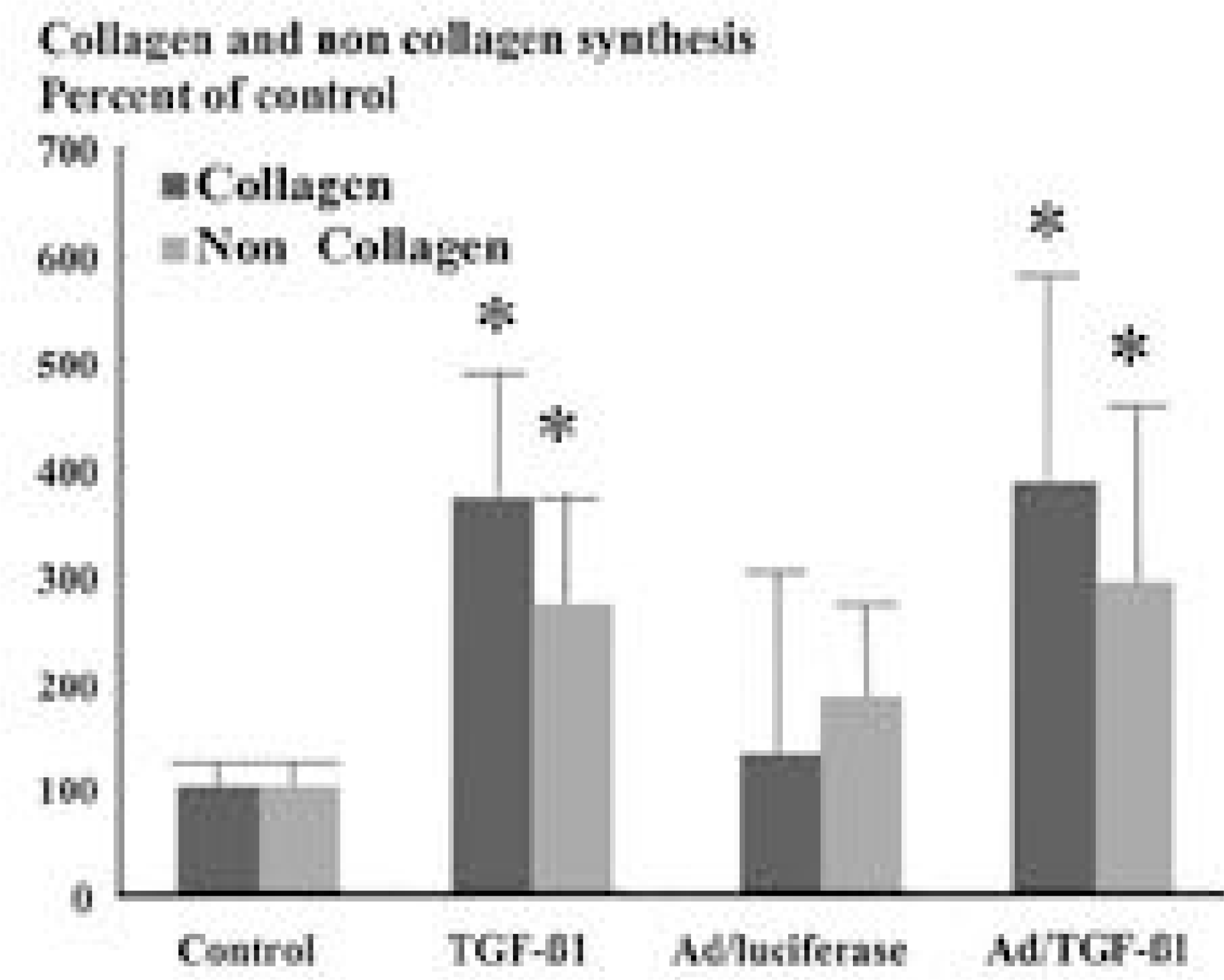 | Fig. 2.Content of newly synthesized collagen and noncollagen as assayed by incorporation of 3H-proline. Human inter-vertebral disc cells transduced by adenovirus-TGFβ1 construct(150 MOI) and thoses treated by TGF-β1 (2 ng/ml) showed 3.5 fold increase in collahen synthesis and 2.5 fold increase in noncollagen synthesis∗(p<0.05). |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download