Abstract
Study Design
This is a retrospective study Objectives: This study compared the clinical outcomes of posterior lumbar interbody fusion (PLIF) using hydroxyapatite blocks with PLIF using a metal or poly-ether-ether-ketone (PEEK) cage. Summary of the Literature Review: There are few reports on the clinical outcomes of PLIF using a hydroxyapatite block for treating lumbar degenerative disease.
Materials and Methods
The 27 PLIF cases (62 units, HA block) that were followed up for 1-year were compared with 13 cases using a metal cage and 13 cases using a PEEK cage. Pedicle screw fixation was performed for all the cases. If the local bone is deficient, then an additional bone graft with autogeous iliac bone or bone substitute was used. The visual analog scale(VAS) for low back pain and radiating pain, the Oswestry disability index (ODI), the intervertebral height and the halo sign around the cages and pedicle screws were comparatively analyzed.
Results
The mean VAS score for low back pain before PLIF and using the HA block, the metal cage and the PEEK cage was 7.5, 8.3 and 6.2, respectively, and this was 3.3, 2.9 and 4.8 after PLIF (P<0.05 with using the HA block and the metal cage (Wilcoxon test). The mean VAS score for radiating pain before PLIF was 7.9, 8.3 and 8.5, respectively, and the VAS score was 3.5, 3.1 and 3.9, respectively, after PLIF (P<0.05 for all cases, Wilcoxon test). For the ODI, the means before PLIF were 60.3, 51.2 and 53.8, respectively, and they changed to 30.5, 24.9 and 29.7, respectively, after PLIF (P<0 .05 for all cases, Wilcoxon test). On the X-ray images, there was no halo sign greater than 2 mm near the pedicle screws or greater than 1 mm near the cages and no breakage of the HA block. No additional bone graft was needed for the PLIF using the HA block and local bone. There was no statistically significant differences among the groups (P< 0.05, One-way ANOVA).
Go to : 
REFERENCES
01). Cloward RB. The treatment of ruptured intervertebral discs by vertebral body fusion. Indication, operative techniques and aftercare. J Neurosurg. 1953. 10:154–168.
02). Enker P., Steffee AD. Interbody fusion and instrumentation. Clin Orthop Relat Res. 1994. 300:90–101.

04). Hioki A., Miyamoto K., Kodama H, et al. Two-level posterior lumbar interbody fusion for degenerative disc disease: improved clinical outcome with restoration of lumbar lordosis. Spine J. 2005. 5:600–607.

05). Madan S., Boeree NR. Outcome of posterior lumbar interbody fusion versus posterolateral fusion for spondylolytic spondylolisthesis. Spine. 2002. 27:1536–1542.

06). Spivak JM., Hasharoni A. Use of hydroxyapatite in spine surgery. Eur Spine J. 2001. 10:197–204.

07). Pope MH., Hanley EN., Matteri RE., Wilder DG., Fry-moyer JW. Measurement of Intervertebral Disc Space Height. Spine. 1977. 2:282–286.

08). Kim JH., Kim SS., Kim JH., Kim BJ. Comparison of monosegment instrumented posterior lumbar interbody fusion with and without a metal cage in degenerative spine. J Korean Orthop Assoc. 2008. 43:143–151.

09). Brantigan JW., Steffee AD. A carbon fiber implant to aid interbody fusion. Two-year clinical results in the first 26 patients. Spine. 1993. 18:2106–2107.
10). Brantigan JW., Steffee AD., Geiger JM. A carbon fiber implant to aid interbody fusion. Mechanical testing. Spine. 1991. 16:277–282.
11). Briggs H., Milligan PR. Chip fusion of the low back following exploration of the spinal canal. J Bone Joint Sug Am. 1944. 26:125–130.
12). Ma GW. Posterior lumbar interbody fusion with specialized instruments. Clin Orthop Relat Res. 1985. 193:57–63.

13). McAfee PC. Interbody fusion cages in reconstructive operation on the spine. J Bone Joint Sug (Am). 1999. 81:859–880.
14). Kim Y., Kang DH., Kim TW. A Study on the Biomechanical Behavior of PLIF using PEEK cages. Transactions of the KSME. 2007. 1:7–11.
15). Be′ker DK., Schulthei� R., van Roost D., Osborn JF., Kaden B. Anterior cervical disectomy and vertebral interbody fusion with Hydroxyl-Apatite ceramic. Preliminary results. Acta Neurochir. 1993. 121:191–195.
16). Bruneau M., Nisolle JF., Gilliard C., Gustin T. Anterior cervical interbody fusion with hydroxyapatite graft and plate system. Neurosurgery Focus. 2001. 10:8.

17). Suetsuna F., Yokoyama T., Kenuka E., Harata S. Anterior cervical fusion using porous hydroxyapatite ceramics of cervical disc herniation. A two-year follow-up. Spine J. 2001. 1:348–357.
18). Haro H., Maekawa S., Hamada Y. Prospective analysis of clinical evaluation and self-assessment by patients after decompression surgery for degenerative lumbar cannal stenosis. The Spine journal. 2008. 8:380–384.
19). Banwart JC., Asher MA., Hassanein RS. Iliac crest bone graft harvest donor site morbidity: A statistical evaluation. Spine. 1995. 20:1055–1060.
20). Verlooy J., De Smedt K., Selosse P. Failure of a modified posterior lumbar interbody fusion technique to produce adequate pain relief in isthmic spondylolytic grade I spondylolisthesis patients. A prospective study of 20 patients. Spine. 1993. 18:1491–1495.
22). Hwang CJ., Bae JY., Koo KH, et al. A comparative experimental study of allograft and porous hydroxyapatite as bone substitutes. J Korean Orthop Assoc. 2007. 42:545–552.

23). Sande′n B., Olerud C., Petre′n-Mallmin M., Johansson C., Larsson S. The significance of radiolucent zones surrounding pedicle screws. Definition of screw loosening in spinal instrumentation. J Bone Joint Sug. 2004. 86:457–461.
Go to : 
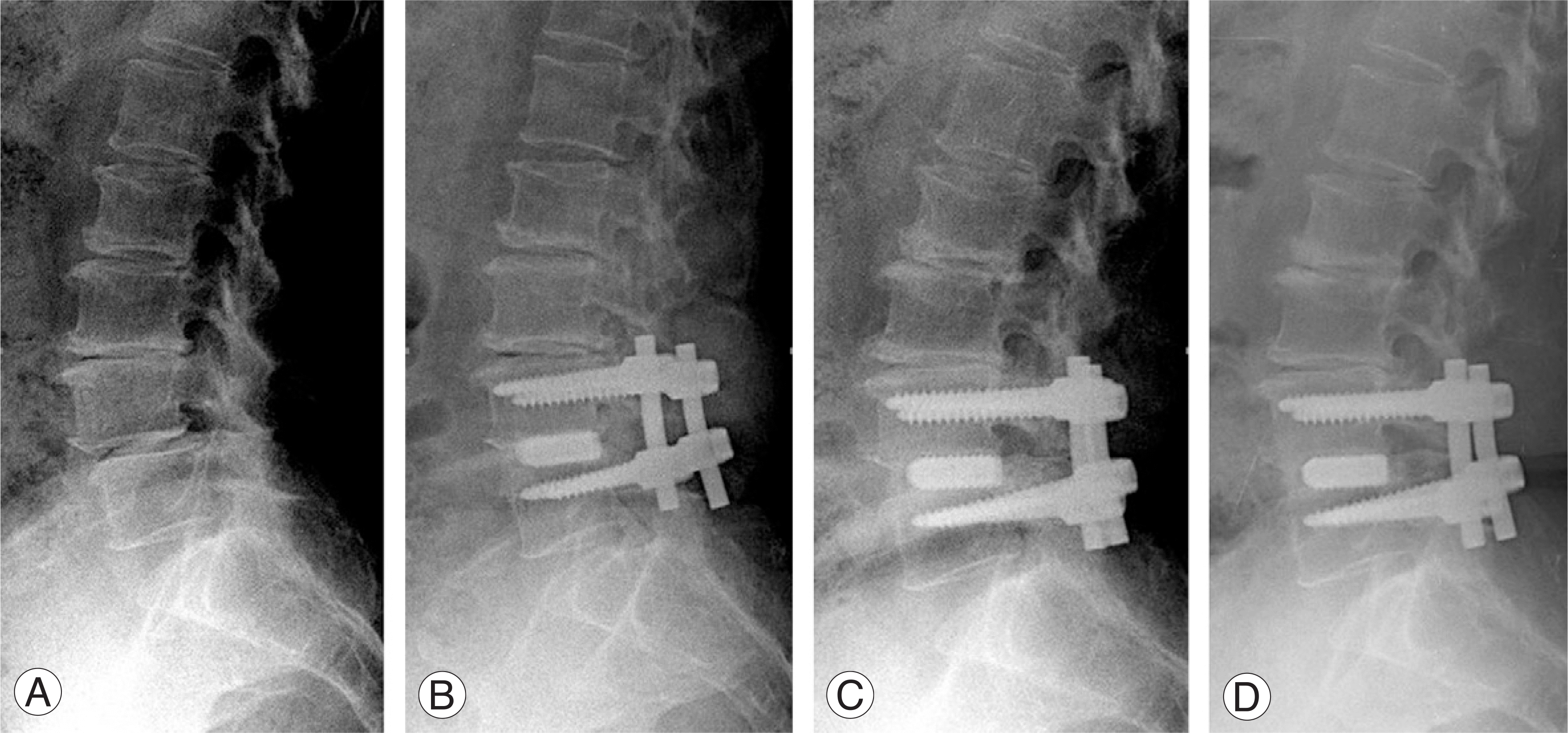 | Fig. 2.The sequential standing lateral radiographs of sixty-six-year-old female patient suffering from spinal stenosis with spondylolisthesis L4 on L5. She was treated with PLIF using local bone grafts and HA blocks. (A) The preoperative lateral radiograph showed a anterior transposition of L4 on L5 vertebral body. (B) The immediately postoperative lateral radiograph shows HA blocks with autogenous local bone graft and pedicle screw stabilization on L4-5. (C) The postoperative lateral radiograph (6 months later) shows bony bridging shadow between L4 and L5 vertebral body and the better visualization of grafted local bone surround HA blocks than initial image. (D) The postoperative lateral radiograph (12 months later) shows a better trabecular bony fusion between L4 and L5 vertebral body and well-maintained HA blocks. |
Table 1.
Classification of fusion results (Brantigan and Steffee, 1991)9,10)
Table 2.
Data of three groups using HA blocks, metal cages, PEEK cages according to the fusion level
| HA block | Metal cage | PEEK cage | |
|---|---|---|---|
| Number of cases | 27 | 13 | 13 |
| 1 level PLIF (%) | 23 (85.2%) | 10 (76.9%) | 10 (76.9%) |
| 2 level PLIF (%) | 4 (14.8%) | 3 (23.1%) | 3 (23.1%) |
| Mean (level) | 1.15 | 1.23 | 1.23 |
Table 3.
Difference of Visual Analogue Scale(VAS) and Oswestry Disability Index(ODI) between preoperativestate and at 1-year follow-up after PLIF surgery
Table 4.
Anteroposterior (AP) disc height at immediate postoperative state and at 1-year follow-up after PILF
| HA block | Metal cage | PEEK cage | Difference between three groups (P-value) | ||||
|---|---|---|---|---|---|---|---|
| IMPO∗ | PO 1Y∗∗ | IMPO∗ | PO 1Y∗∗ | IMPO∗ | PO 1Y∗∗ | ||
| MIH†(mm) | 13.44±1.69 | 13.16±1.71 | 14.90±1.57 | 14.60±1.48 | 12.21±1.48 | 11.89±0.97 | > 0.05 |
| Collapsed MIH† (No. of cases) | 0 | 0 | 0 | ||||




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download