Abstract
Materials and Methods
The operations were performed for 24 patients who were scheduled to undergo posterolateral fusion with internal fixation between January 2004 and March 2006. There were 7 male and 17 female patients, and the average age of the patients was 54 (range: 12~71) years. All fo these patients were indicated for posterolateral fusion with internal fixation requiring intraoperative and postoperative blood transfusion. In the whole study group, all of the patients refused to receive conventional transfusion therapy because of religious convictions. To categorize the patients by disease, there were 17 cases of spinal stenosis, two cases of spondylolytic spondylolisthesis, four cases of spinal stenosis with degenerative spondylolisthesis, and one case of neurofibromatosis scoliosis. In order to increase hemoglobin level, recombinant human erythropoietin was administered before the operation, and controlled hypotensive anesthesia, acute normovolemic hemodilution, a cell saving system, and reducing operating time methods were intraoperatively used to spare blood in spine surgery. Postoperatively, recuperative techniques were used to decrease blood loss and maximize blood production.
Go to : 
REFERENCES
1). Clark JM. Surgery in Jehovwah's witnesses. Br J Hosp Med. 1982; 27:497–500.
2). CDC Update. Acquired immunodeficiency syndrome-Unite state 1989. MMWR. 1990; 39:81–84.
3). Curran JW, Lawrence DN, Jaffe H, et al. .:. Acquired immunodeficiency syndrome (AIDS) associated with transfusion. NEJM. 1984; 310:69–75.
4). Goulet JA, Bray TJ, Timmerman LA, Benson DR, Bargar AWL. Intraoperative autologous transfusion in orthopardic patients. J Bone and Joint Surgery Am. 1989; 71(1):3–8.
5). David C, Mann. Decreasing homologous blood transfusion in spinal surgery by use of the cell caver and predict-ed blood. Spine. 1989; 14:1296–1302.
6). Cavallieri S, Riou B, Roche S, et al. .:. Intraoperative autologous transfusion in emergency surgery for spine trauma. J Trauma. 1994; 36:639–643.

7). Bovill DF, Moulton CW, Jackson WST, Jensen JK. The efficacy of intraoperative autologous transfusion in major orthopedic surgery: A Regression analysis. Orthopedics. 1986; 9:1403–1407.

8). American Association of Blood Bank. Guidelines for blood salvage and reinfusion in surgery and trauma. 1988-1989 and 1989-1990, AABB, autologous transfusion committee.
9). Simpson MB, Georgopoulos G, Eilert RE. Intraoperative blood salvage in children and young adults undergoing spinal surgery with predeposited autologous blood: Efficacy and cost effectiveness. J Prosthet Orthot. 1993; 13:777–780.

10). Flynn JC, Metzgr CR, Csencsitz TA. Intraoperative autotransfusion (IAT) in spinal surgery. Spine. 1982; 7:432.

11). Messer K. Hemodilution. Surg Clin North Am. 1975; 55:659–678.
12). Blundell J. Experiments on the transfusion of blood by syringe. Med Chir Tr. 1818; 9:56–92.
13). Klebanoff G. Early clinical experience with a disposable unit for intraoperative salvage and reinfusion of blood loss (intraoperative autotransfusion). Am J Surg. 1970; 120:718–722.
14). Boudreaux JP, Bornside GH, Cohn I Ir. Emergency autotransfusion; partial cleansing of bacteria-laden blood by cell washing. J Trauma. 1983; 23:31–35.
15). Healy WL, Pfelfer BA, Kurtz SR, et al. .:. Evaluation of autologous shed blood for autotransfusion after orthopaedic surgery. Clin Orthop. 1994; 299:53–58.

16). Roye DP Jr. Recombinant human erythropoietin and blood management in pediatric spine surgery. Orthopedics. 1999; 22:158–160.

17). Coyle D, Lee KM, Fergusson DA, et al. .:. Economic analysis of erythropoietin use in orthopaedic surgery. Transfus Med. 1999; 9:21–30.

18). Lee JH, Lee SH, Oh JH. Minimal effective dosage of recombinant human erthropoietin in spinal surgery. Clin Orthop. 2003; 412:71–76.
19). Mercuriali F, Zanella A, Barsosi G, et al. .:. Use of erthropoietin to increase the volume of autologous blood donated by orthopedic patients. transfusion. 1993; 33:55–60.
Go to : 
Figures and Tables%
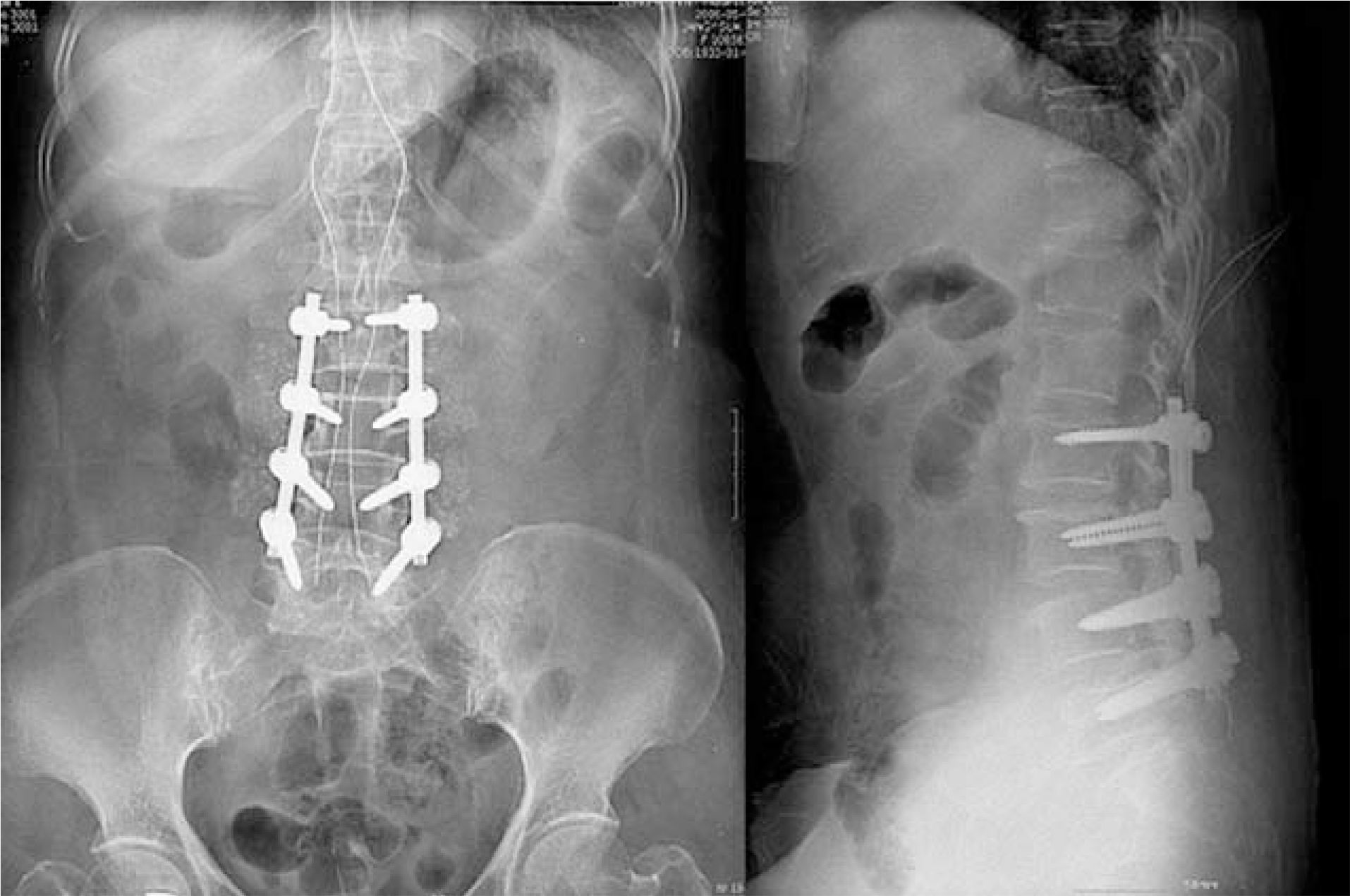 | Fig. 1.Postoperative anteroposterior and lateral plain radiographs show posterior decompression and instrumented posterolateral fusion for spinal stenosis. |
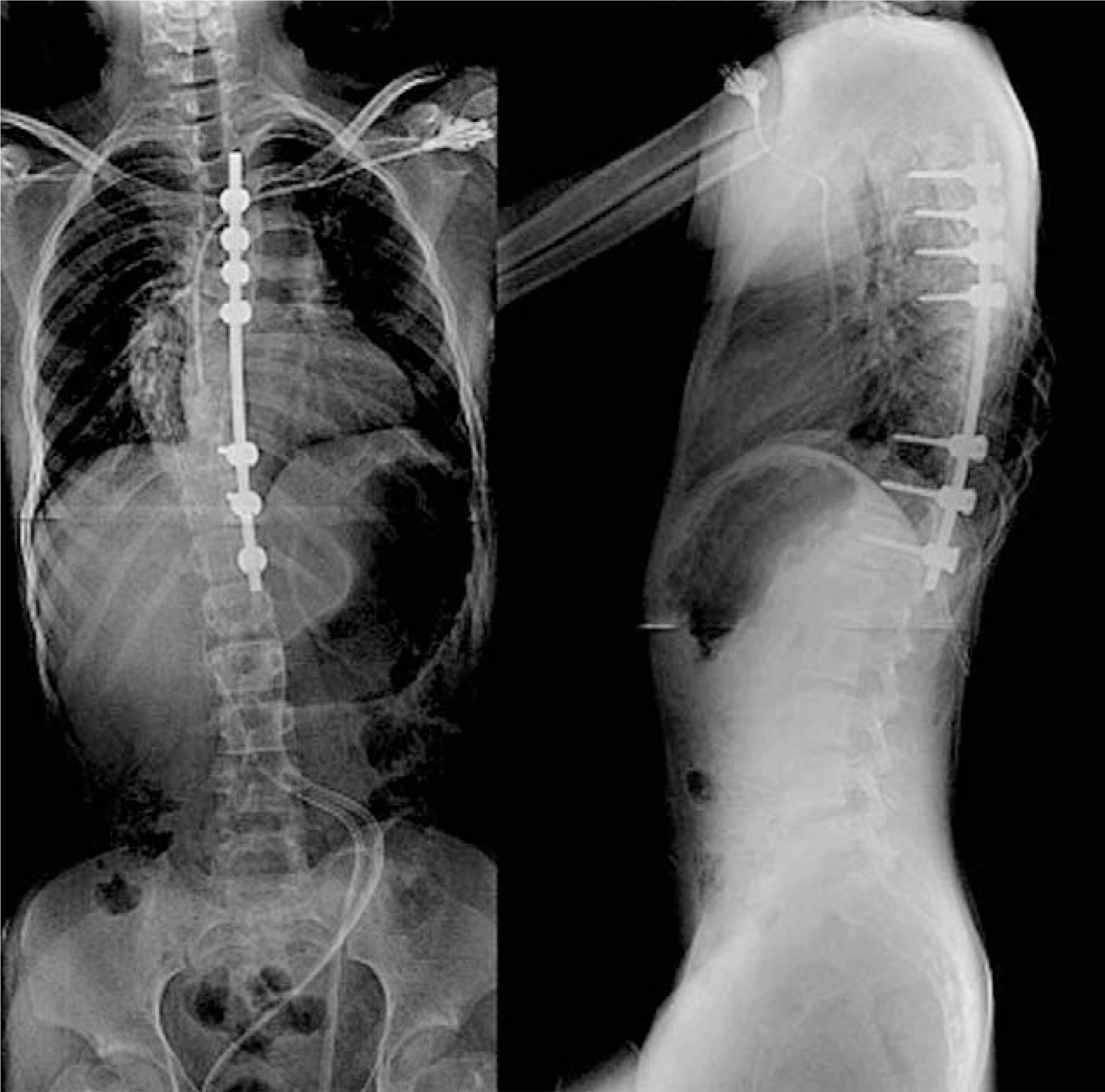 | Fig. 2.Postoperative anteroposterior and lateral plain radiographs show scoliosis correction in neurofibromatosis patient. |
Table 1.
Geographic data about Patient's age, sex, diagnosis and operation name
Table 2.
Geographic data on blood loss, operation time, changes of Hb, selected protocol
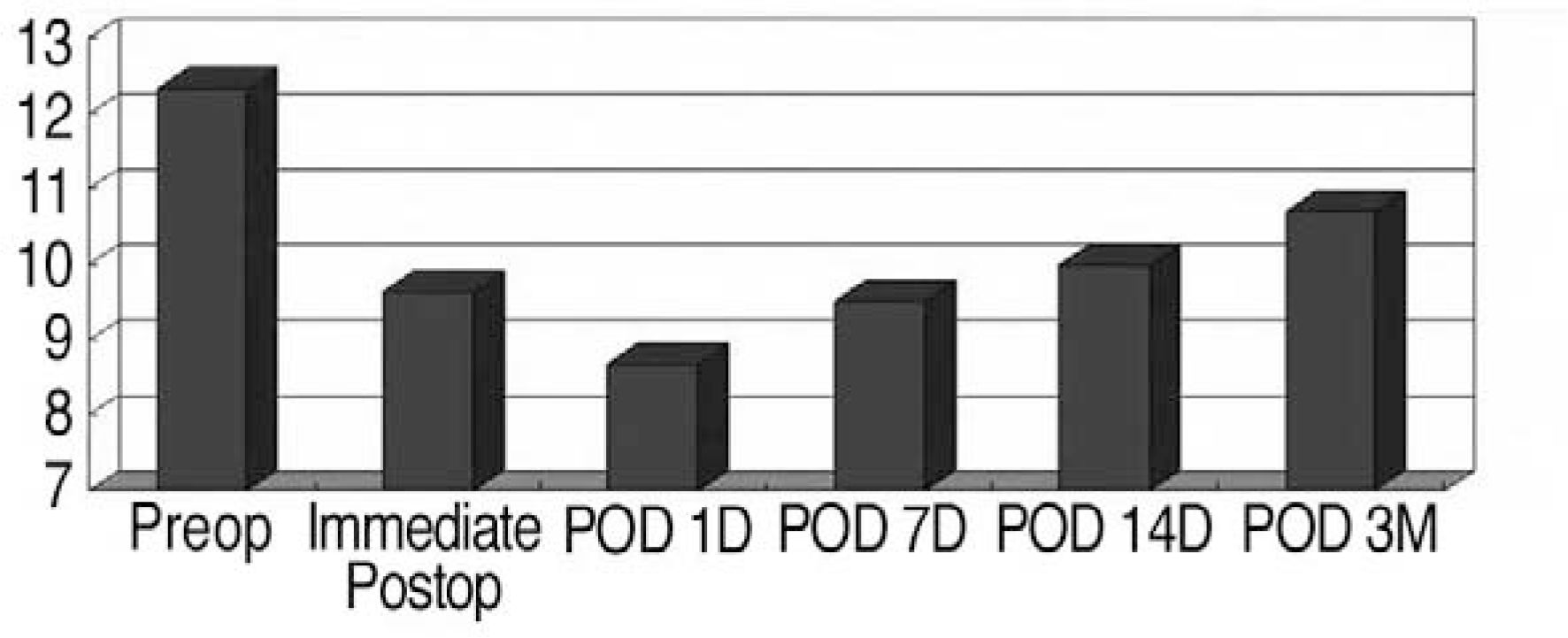
Hb: Hemoglobin (g/dl); EPO2: Recombinant Erythropoietin 2000 unit; EPO4: Recombinant Erythropoietin 4000 unit; Ferr: Ferrum Pola �(Ferric hydroxide-polymaltose complex 357 mg, Folic acid 350 mcg); ANH: Acute normovolemic hemodilution; CS: Cell saver; Veno: Venoferrum � (Ferric hydroxide sucrose complex)
Table 3.
Protocol for pre and postoperation
Table 4.
Drugs for pre and postoperation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download