Abstract
Objectives
To examine the ability of human umbilical cord blood (hUCB) stem cells to target a zone of injury and to determine the efficacy of hUCB cells to ameliorate the behavioral deficits after a hUCB cell infusion in paralyzed rats.
Summary of Literature
Many groups have investigated the use of stem cells as potential treatments for a CNS injury. hUCB cells have recently been reported to alleviate the behavioral consequences of a stroke injury.
Materials and Methods
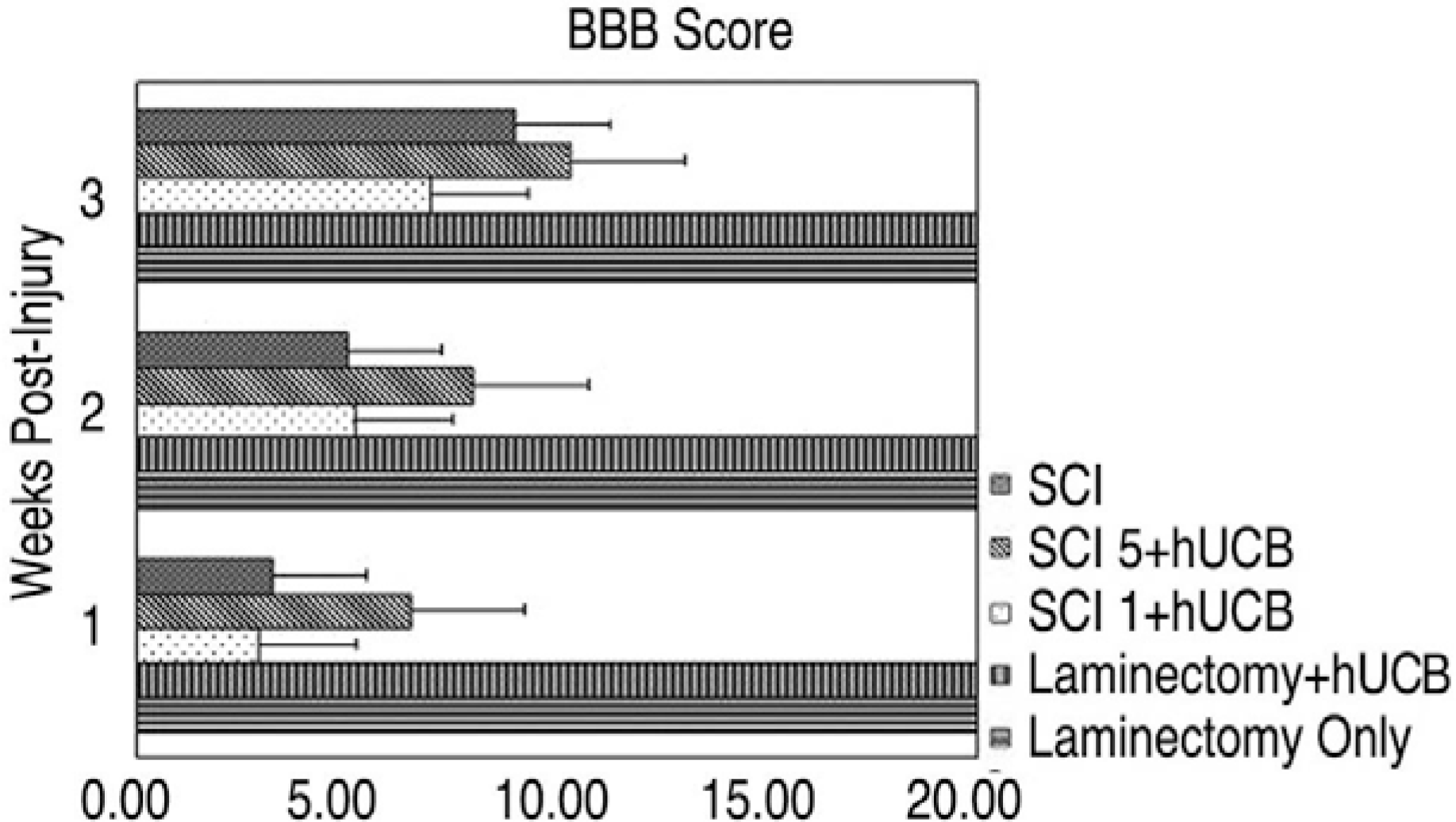
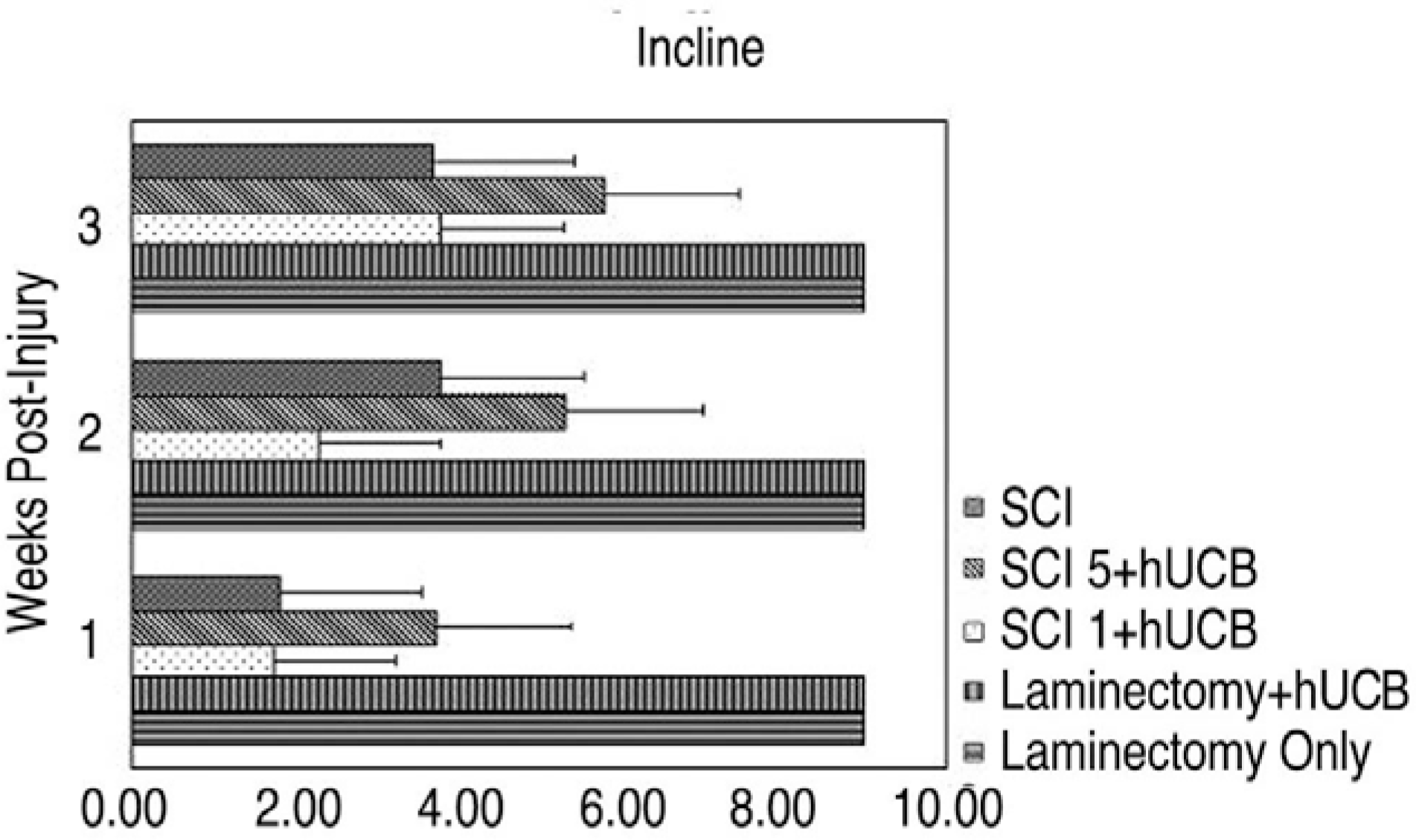
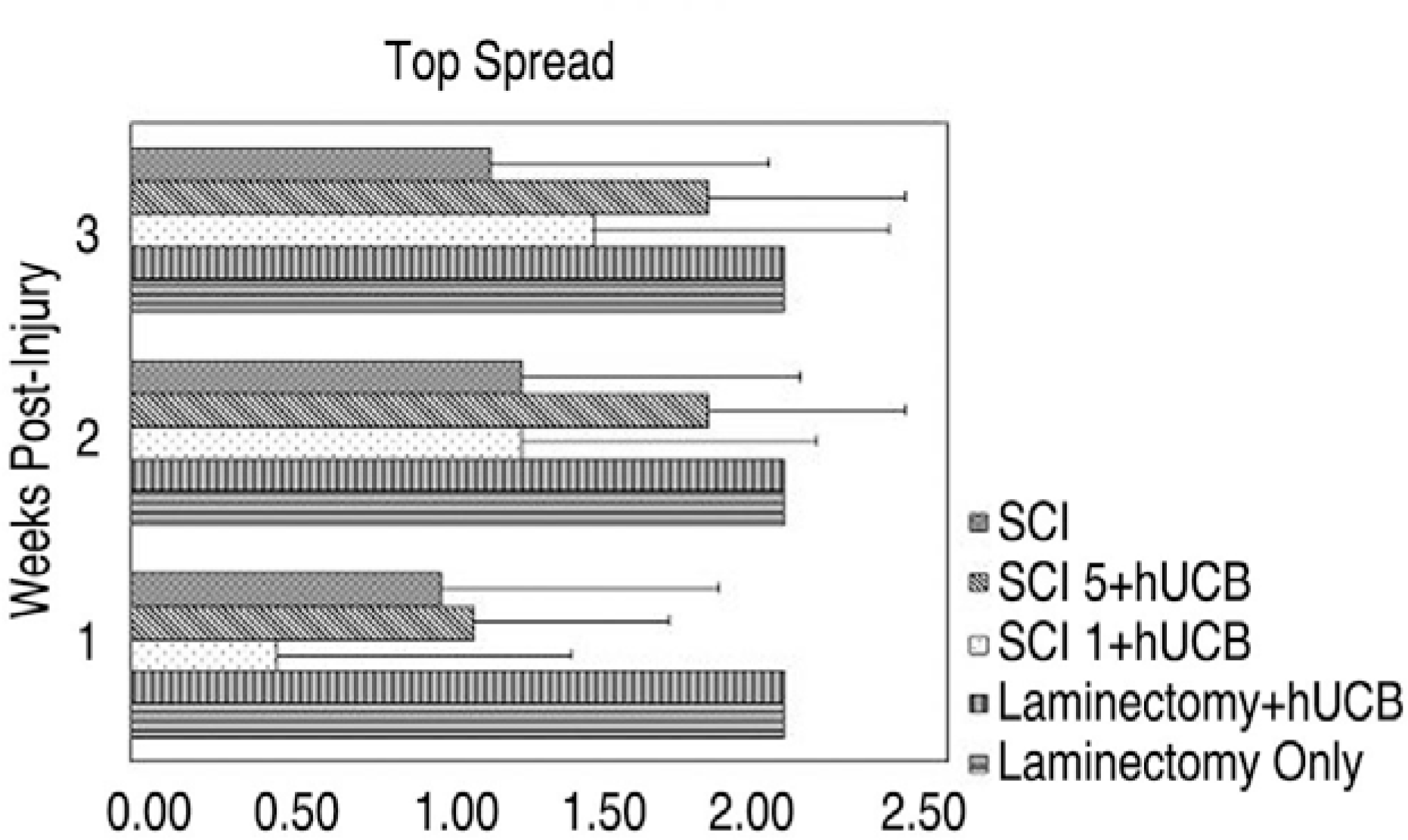
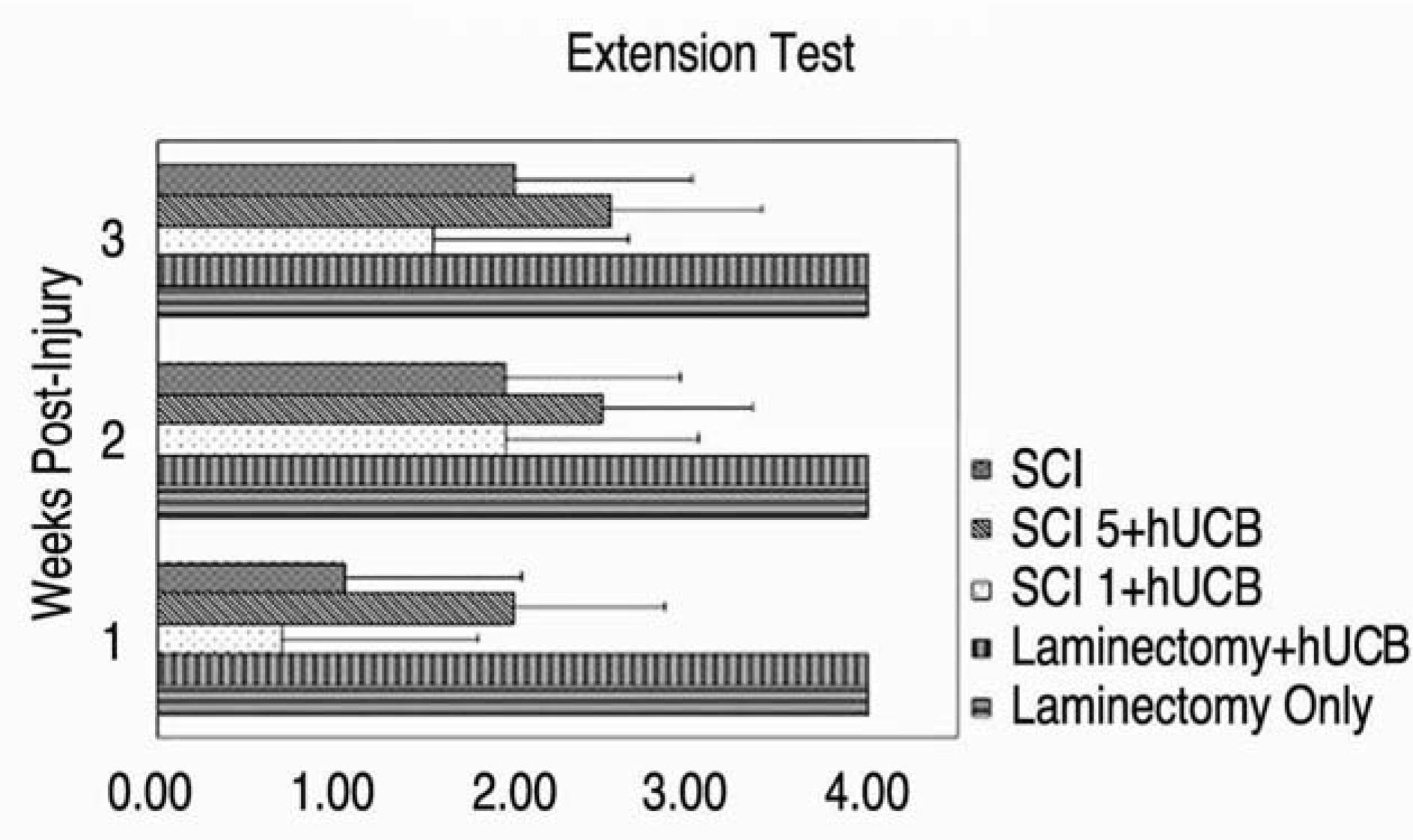
Thirty Sprague Dawley rats were divided into 6 groups (Gr) (Gr 1. SCI (spinal cord injury) + hUCB delivered at one day postinjury, Gr 2. SCI + hUCB delivered at 3 days postinjury, Gr 3. SCI + hUCB delivered at 5 days postinjury, Gr 4. laminectomy + hUCB, Gr 5. SCI only, Gr 6. Laminectomy only). SCI was produced by compressing the spinal cord to the level of the 8-9th thoracic spine for 1 minute with an aneurysm clip that was calibrated to a closing pressure of 50 gms. The hUCB cells (0.5 ml, 1.5×106) were administered intravenously to the rats. The rat was assessed behaviorally at one, two and three weeks using the BBB behavioral scale. Four weeks after the injury, the animals were sacrificed and the hUCB positive-response neural cells (mouse anti-human mitochondria monoclonal antibody=MAB 1273) at the injury level observed using optical and fluorescent microscopy.
Results
MAB 1273 positive cells were observed in groups 1, 2 and 3 but not in groups 4, 5 and 6. In particular, there were 870 cells distributed over an area of 1.2 mm2 in group 3. Group 3 showed the most significant recovery over time in the open field exam, and the most improvement in another tests of incline, leg extension, and toe spread compared with group 1 (p�0.01).
Go to : 
REFERENCES
1). Nobunaga AI, Go BK, Karunas RB. Recent demograph-ic and injury trends in people served by the Model Spinal Cord Injury Care Systems. Arch Phys Med Rehabil. 1999; 80:1372–1382.

2). Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999; 19:5810–5822.

3). Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001; 11:89–94.

4). Kwon BK, Borisoff JF, Tetzlaff W. Molecular targets for therapeutic intervention after spinal cord injury. Mol Interv. 2002; 2:244–258.

5). Kwon BK, Fisher CG, Dvorak MF, Tetzlaff W. Strate-gies to promote neural repair and regeneration after spinal cord injury. Spine. 2005; 30:S3–13.

6). Lu D, Sanberg PR, Mahmood A, Li Y, Wang L, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002; 11:275–281.

7). Duchossoy Y, Kassar-Duchossoy L, Orsal D, Stettler O, Horvat JC. Reinnervation of the biceps brachii muscle following cotransplantation of fetal spinal cord and autol-ogous peripheral nerve into the injured cervical spinal cord of the adult rat. Exp Neurol. 2001; 167:329–340.

8). Liu S, Qu Y, Stewart TJ, et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci U S A. 2000; 97:6126–6131.

9). Kimura H, Yoshikawa M, Matsuda R, et al. Transplantation of embryonic stem cell-derived neural stem cells for spinal cord injury in adult mice. Neurol Res. 2005; 27:812–819.

10). Eckfeldt CE, Mendenhall EM, Verfaillie CM. The molecular repertoire of the ‘ almighty'stem cell. Nat Rev Mol Cell Biol. 2005; 6:726–737.
11). Saporta S, Kim JJ, Willing AE, Fu ES, Davis CD, Sanberg PR. Human umbilical cord blood stem cells infusion in spinal cord injury: engraftment and beneficial influence on behavior. Hematother Stem Cell Res. 2003; 12:271–278.

12). Willing AE, Lixian J, Milliken M, et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. Neurosci Res. 2003; 73:296–307.

13). Lu D, Sanberg PR, Mahmood A, et al. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002; 11:275–281.

14). Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995; 12:1–21.

15). Zeman RJ, Feng Y, Peng H, et al. X-irradiation of the contusion site improves locomotor and histological outcomes in spinal cord-injured rats. Exp Neurol. 2001; 172:228–234.

16). Pranke P, Failace RR, Allebrandt WF, Steibel G, Schmidt F, Nardi NB. Hematologic and immunopheno- typic characterization of human umbilical cord blood. Acta Haematol. 2001; 105:71–76.
17). von Euler M, Seiger A, Sundstrom E. Clip compression injury in the spinal cord: a correlative study of neurological and morphological alterations. Exp Neurol. 1997; 145:502–510.

18). Chen N, Hudson JE, Walczak P, et al. Human umbilical cord blood progenitors: the potential of these hematopoietic cells to become neural. Stem Cells. 2005; 23:1560–1570.

19). Walczak P, Chen N, Hudson JE, et al. Do hematopoietic cells exposed to a neurogenic environment mimic properties of endogenous neural precursors? J Neurosci Res. 2004; 76:244–254.

20). Ourednik J, Ourednik V. Graft-induced plasticity in the mammalian host CNS. Cell Transplant. 2004; 13:307–318.

Go to : 
Figures and Tables%
 | Fig. 1.Identification of hUCB in compressed spinal cord immunohistochemical stained with mouse anti-human mitochondria monoclonal antibody (MAB1273). (A) hUCB were injected into the tail vein 1 day compression injury. We can observed immunopositive hUCB for the MAB1273 (arrows). (C) hUCB were injected into the tail vein 3 days compression injury. We could observed numerous immunopositive hUCB for the MAB1273 (arrows). (E) hUCB cells were injected into the tail vein 5 days compression injury. We can observed numerous immunopositive hUCB for the MAB1273 (arrows). (B, D, F) (right panel) were of the contralateral spinal cord, in which immuno-positiv hUCB were not found. |
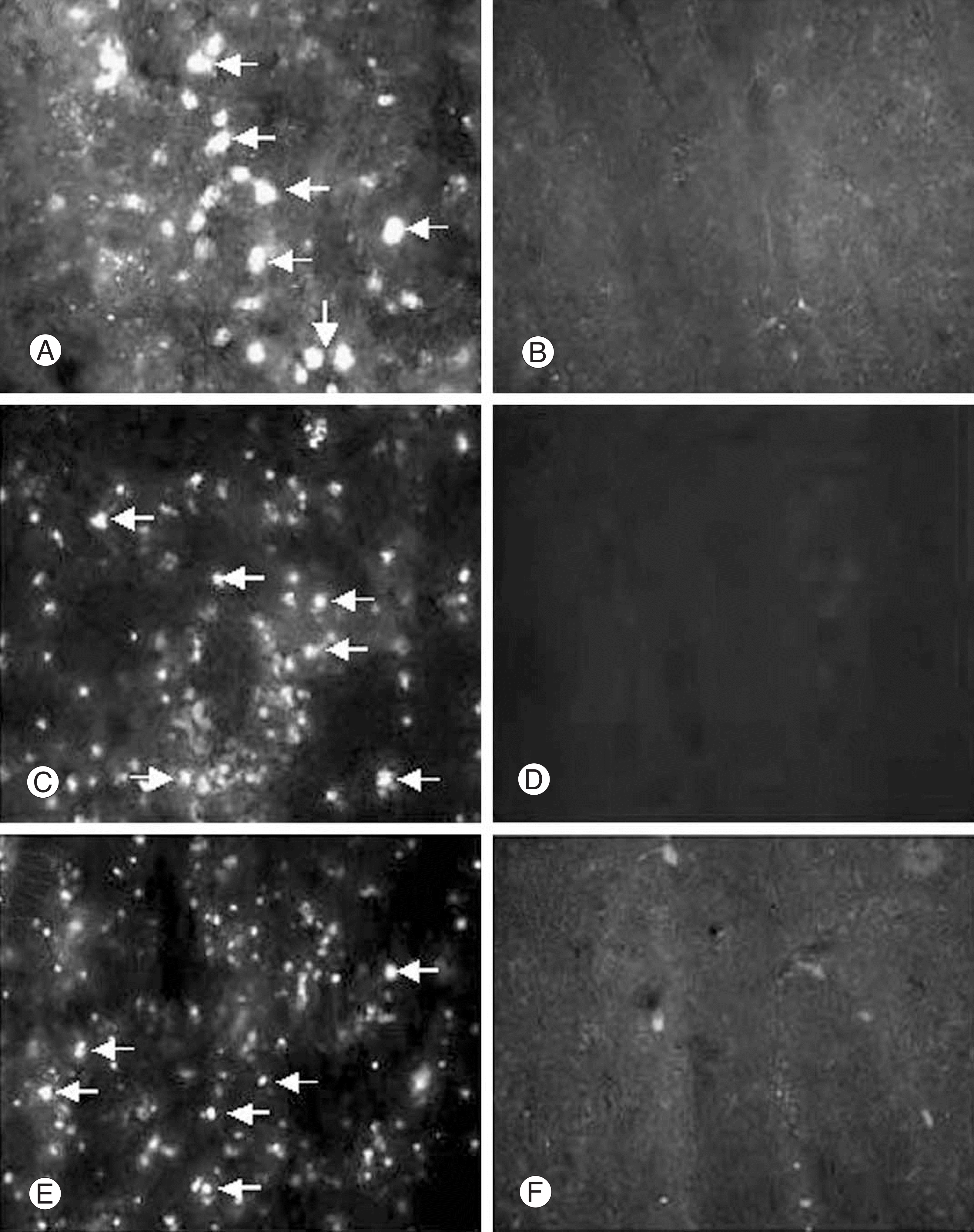 | Fig. 2.Identification of the hUCB in compressed spinal cord. hUCB, prelabeled with FITC-conjugated cholera toxin, were injected into the tail vein 1, 3, 5 days after compression injury. Fluorescent hUCB (MAB1273, arrows) are seen within the injured spinal cord at 5(A), 3(C), 1(E) days post-injection. Right panel (B, D, F) is of the contralateral spinal cord (intact spinal cord), in which fluorescent hUCB were not found. |
Table 1.
행동평가




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download