Abstract
Objectives
To compare the efficacy of demineralized bone matrix as a bone graft extender in lumbar posterolateral fusion with cases using an autogenous iliac bone graft.
Summary of Literature Review
Since demineralized bone grafts were introduced for bone graft extension in 1995, many types of demineralized bone matrices have been used with improved fusion rates.
Materials and Methods
From October 2004 to December 2005, demineralized bone matrices were used as iliac bone graft extenders in 49 cases (Group I) of lumbar posterolateral fusion, compared with 50 cases receiving autogenous grafts (Group II) similar in age, bone marrow density, and number of fusion levels. Fusion status was graded by the Lenke classification and data was analyzed using a chi-square test through SPSS v.10.0.
Go to : 
REFERENCES
1). Lee YP, Jo M, Luna M, Chien B, Lieberman JR, Wang JC. The efficacy of different commercially available demineralized bone matrix substances in an athymic rat model. J Spinal Disord Tech. 2005; 18:439–444.

2). Lenke L, Bridwell K, Bullis D, Betz R, Baldus C, Schoenecker C. Results of in situ fusion for isthmic spondylolisthesis. J Spinal Disord. 1992; 5:433–443.

3). Sanden B, Orelud C, Petren-Malmin M, Johansson C, Larsson S. The significance of radiolucent zones surrounding pedicle screws. J Bone Joint Surg Br. 2004; 86:457–461.
4). Lee KJ, Roper JG, Wang JC. Demineralized bone matrix and spinal arthrodesis. Spine J. 2005; 5:217–223.

6). Cammisa FP Jr, Lowery G, Garfin SR, et al. Two-year fusion rate equivalency between Grafton DBM gel and autograft in posterolateral spine fusion: a prospective controlled trial employing a side-by-side comparison in the same patient. Spine. 2004; 29:660–666.
7). Gigardi FP, Cammisa FP Jr. The effect of bone graft extenders to enhance the performance of iliac crest bone grafts in instrumented lumbar spinal fusion. Orthopedics. 2003; 26:545–548.

8). Price CT, Connolly JF, Carantzas AC, Ilyas I. Comparison of bone graft for posterior spinal fusion in adolescent idiopathic scoliosis. Spine. 2003; 28:793–798.
9). Vaccaro AR, Chiba K, Heller JG, et al. Bone grafting alternatives in spinal surgery. Spine J. 2002; 2:206–215.

10). Cook SD, Dalton JE, Prewett AB, Whitecloud TS 3rd. In vivo evaluation of demineralized bone matrix as a bone graft substitute for posterior spinal fusion. Spine. 1995; 20:877–886.

11). Helm GA, Sheehan JM, Sheehan JP, et al. Utilization of type I collagen gel, demineralized bone matrix, and bone morphogenetic protein-2 to enhance autologous bone lumbar spinal fusion. J Neurosurg. 1997; 86:93–100.

12). McLain RF, Fleming JE, Boehm CA, Muschler GF. Aspiration of osteoprogenitor cells for augmenting spinal fusion: comparison of progenitor cell concentration from the vertebral body and iliac crest. J Bone Joint Surg Am. 2005; 87:2655–2661.
13). Lidsey RW, Wood GW, Sadasivian KK, Stubbs HA, Block JE. Grafting long bone fractures with demineralized bone matrix putty enriched with bone matrix: pilot findings. Orthopedics. 2006; 29:939–941.
14). Qiu QQ, Shih MS, Stock K, et al. Evaluation of DBM/AM composite as a graft substitute for posterolateral lumbar fusion. J Biomed Mater Res B Appl Biomater 2006;Dec 20: Epub ahead of print.
15). Wang JC, Alanny A, Mark D, Kanim LE, et al. A comparison of commercially available demineralized bone matrix for spinal fusion: Eur Spine J. 2007; 16:1233–1240.
Go to : 
Figures and Tables%
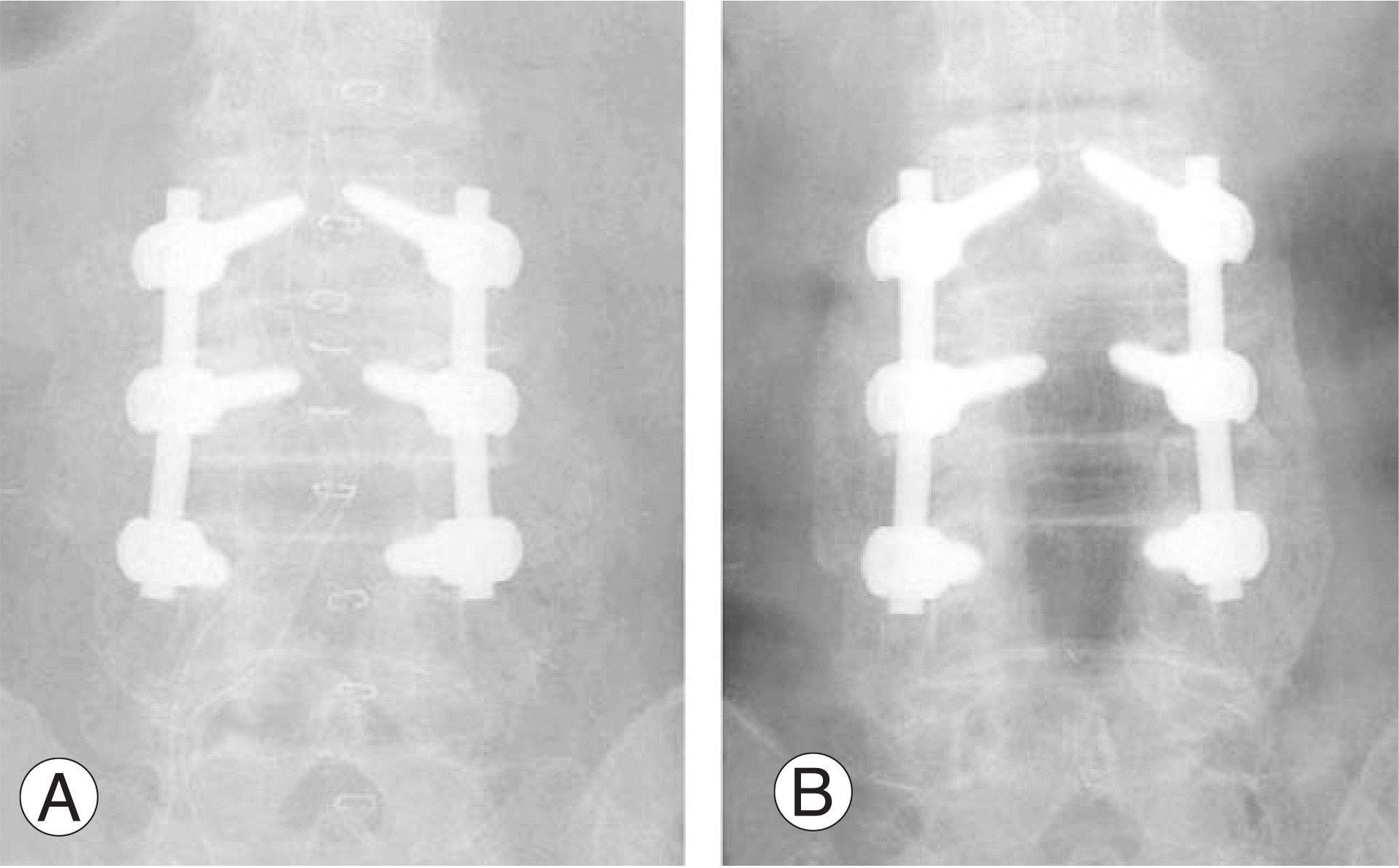 | Fig. 1.(A) Postoperative x-ray of 82-year-old female patient who is grafted using Grafton demineralized bone matrix. (B) Bilateral thick and solid fusion masses are seen in posteoperative 1 year and classified as Lenke A. |
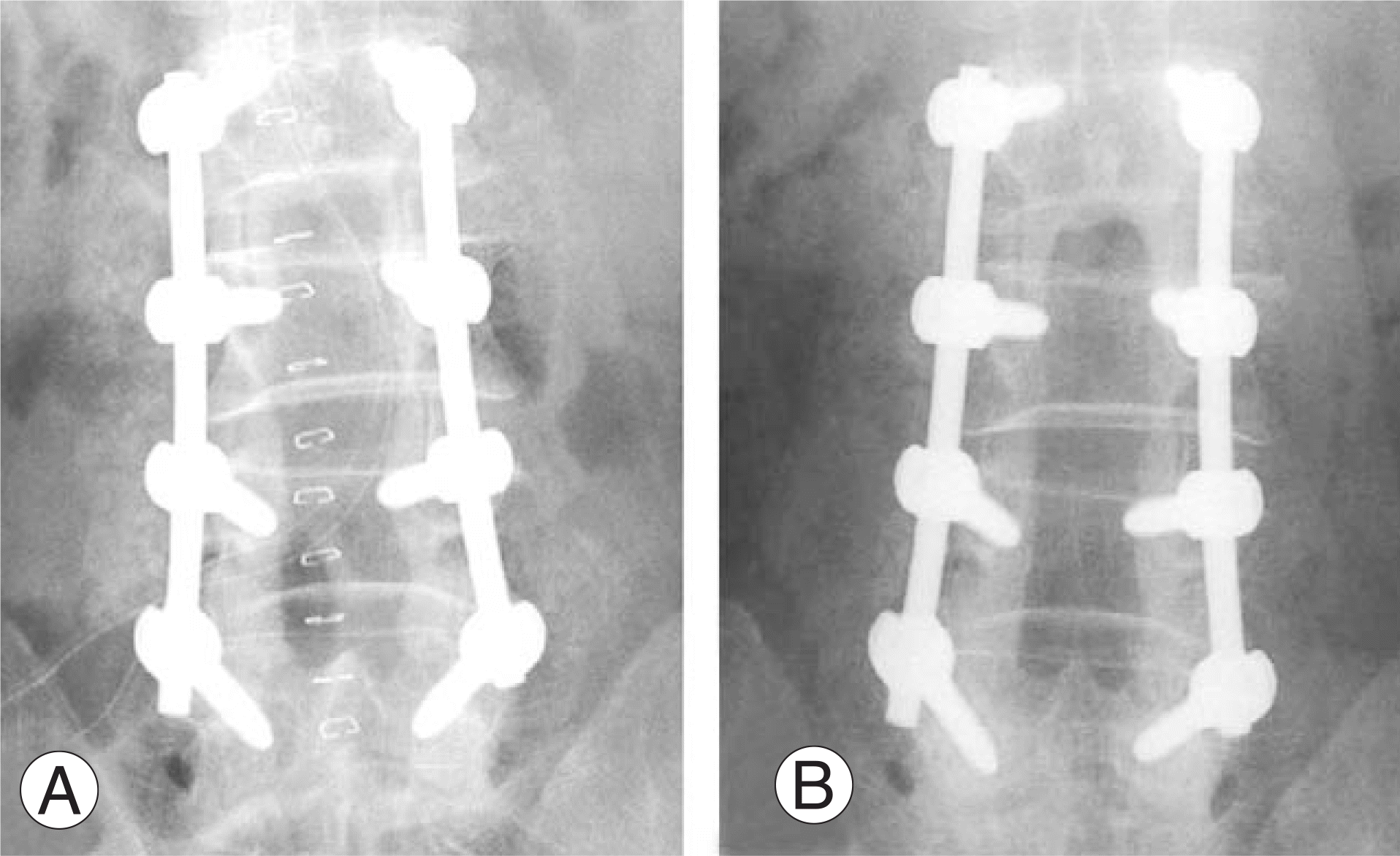 | Fig. 2.(A) 62-year-old male patient's postoperative X-ray shows that Orthoblast II was used with laminar fragmented bone which was removed during decompression. (B) Grafted demineralized bone matrix and autogenous laminar bone are disappeared and radiolucent zone surrounding pedicle screws are seen. Lenke classification D. |
Table 1.
Patients using demineralized bone matrix
Table 2.
Fusion status according to number of fusion segments




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download