Abstract
Objectives
The aim of this study was to evaluate the efficacy and safety of Ultracet TM compared with a placebo in the treatment of acute pain after spinal surgery.
Summary of Literature Review
Ultracet TM is a combination drug of Tramadol and Acetaminophen, and the synergistic effect in pain control was demonstrated by animal experiments.
Materials and Methods
Seventy-six patients who satisfied the selection and exclusion criteria after spinal surgery were enrolled in this study. The patients measured perceptible pain relief time and meaningful pain relief time using a two stop-watch technique. The pain intensity (PI) and pain relief (PAR) were recorded at 30 minutes and then hourly over a 4 hour period, and the pain intensity difference (PID), the sum of the pain intensity difference (SPID), and the total pain relief (TOPAR) were also checked.
Results
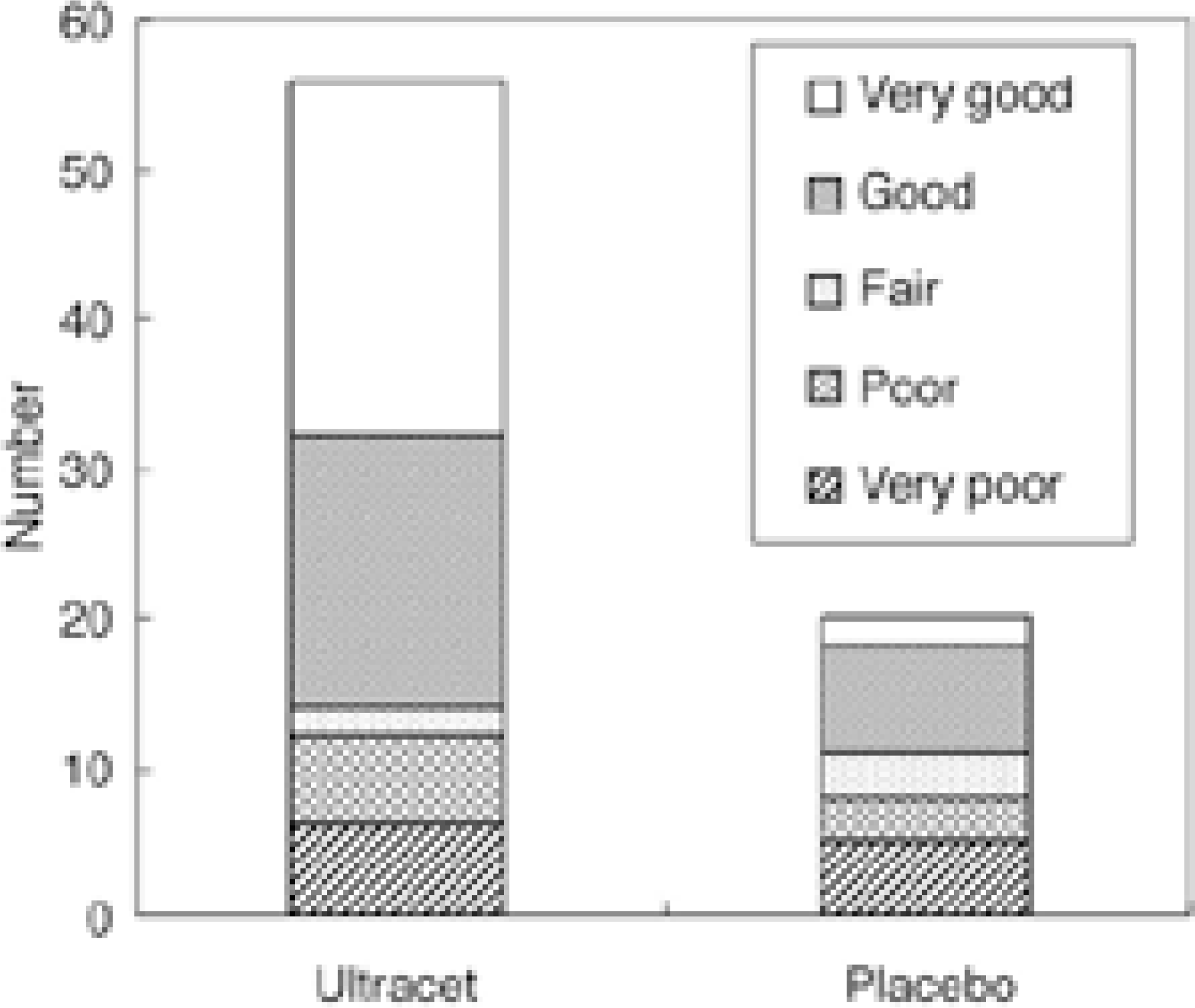
The study and control group comprised of 56 and 20 patients, respectively. The baseline pain intensity was an average of 5.9±1.2 in the study group and 6.1±1.4 in the control group (p=0.683). The final pain intensity was 2.5±2.4 and 4.1±2.2 in the study and control group, respectively. The study group was superior to placebo (p=0.008). In addition, the study group was statistically superior in terms of the PID (p=0.025), SPID (p=0.028), and TOPAR (p=0.048), particularly over 2 hours, as well as the overall assessment (p=0.005). The median time of the meaningful pain relief time was 90 and 193 minutes in the study and control group, respectively.
Conclusions
The analgesic efficacy of Ultracet TM was superior to the placebo on the SPID, TOPAR, and the subjects’ overall assessments over the 4 hour observation period. These results suggest that UltracetTM is an effective therapeutic option for the management of acute pain after spinal surgery without serious complications.
Go to : 
REFERENCES
1). Merskey H, Bogduk N. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. IASP press: Seattle;1994.
2). Barkin RL. Acetaminophen, aspirin, or Ibuprofen in combination analgesic products. Am J Ther. 2001; 8:433–442.

4). Desmeules J, Rollason V, Piguet V, dayer P. Clinical pharmacology and rati-onale of anagesic combinations. Eur J Anaesthesiol Suppl. 2003; 28:7–11.
5). Lo´pez-Mun˜ oz FJ, Salazar LA. Determination of analgesic interaction between acetaminophen and d-propoxyphene obtained by means of the surface of synergistic interaction. Methods Find Exp Clin Pharmacol. 1995; 17:311–320.
6). Raffa RB. Pharmacology of oral combination analgesics: rational therapy for pain. J Clin pharm Ther. 2001; 26(4):257–264.

7). Schnitzer T. The new analgesic combination tramadol/acetaminophen. Eur J Anaesthesiol Suppl. 2003; 28:13–17.
8). Tallarida RJ, Raffa RB. Testing for synergism over a range of fixed ratio drug combinations: Replacing the isobologram. Life Sci. 1996; 58:23–28.

9). Bjo¨rkman R. Central antinociceptive effects of non-steroidal antiinflammatory drugs and paracetamol. Experimental studies in the rat. Acta Anaesthesiol Scand Suppl. 1995; 39(S103):1–44.
10). Bjo¨rkman R, Hallman KM, Hedner J, Hedner T, Hen-ning M. Acetaminophen blocks spinal hyperalgesia induced by NMDA and substance P. Pain. 1994; 57:259–264.
12). Hunskaar S, Fasmer OB, Hole K. Acetylsalicylic acid, paracetamol and mor-phine inhibit behavioral responses to intrathecally administered substance P or capsaicin. Life Sci. 1985; 37:1835–1841.

13). Muth-Selbach US, Tegeder I, Brune K, Geisslinger G. Acetaminophen inhibits spinal prostaglandin E2 release after peripheral noxious stimulation. Anesthesiol. 1999; 91:231–239.
14). Pelissier T, Alloui A, Paeile C, Eschalier A. Evidence of a central anti-nociceptive effect of paracetamol involving spinal 5HT3 receptors. Neuroreport. 1995; 6:1546–1548.

15). Halfpenny DM, Callado LF, Stamford JA. Is tramadol an antidepressant? Br J Anaesthesiol. 1999; 82:480–481.

16). Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992; 260:275–285.
17). Raffa RB, Friderichs E, Reimann W, et al. .:. Comple-mentary and synergistic anti-nociceptive interaction between the enantiomers of tramadol. J Pharmacol Exp Ther. 1993; 267:331–340.
18). Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med. 2003; 114:537–545.

19). Fricke JR Jr, Karim R, Jordan D, Rosenthal N. A dou-ble-blind, single-dose comparison of the analgesic efficacy of tramadol/acetaminophen combination tablets, hydrocodone/acetaminophen combination tablets, and placebo after oral surgery. Clin Ther. 2002; 24:953–968.

20). Fricke JR Jr, Hewitt DJ, Jordan D, Fisher A, Rosenthal N. A double-blind placebo-controlled comparison of tramadol/acetaminophen and tramadol in patients with postoperative dental pain. Pain. 2004; 109:250–257.

21). Jung YS, Kim DK, Kim MK, et al. .:. Onset of analgesia and analgesic efficacy of tramadol/acetaminophen and codeine/acetaminophen/ibuprofen in acute postoperative pain: a single-center, single-dose, randomized, active-controlled, parallel-group study in a dental surgery pain model. Clin Ther. 2004; 26:1037–1045.

22). McQuay H, Edwards J. Meta-analysis of single dose oral tramadol plus acetaminophen in acute postoperative pain. Eur J Anaesthesiol Suppl. 2003; 28:19–22.
23). Medve RA, Wang J, Karim R. Tramadol and acetaminophen tablets for dental pain. Anesth Prog. 2001; 48:79–81.
24). Peloso PM, Fortin L, Beaulieu A, Kamin M, Rosenthal N. Analgesic efficacy and safety of tramadol/acetaminophen combination tablets (Ultracet) in treatment of chronic low back pain: a multicenter, outpatient, randomized, double blind, placebo controlled trial. J Rheumatol. 2004; 31:2454–2463.
25). Rosenthal N, Silverfield JC, Wu SC, Jordan D, Kamin M. Tramadol/acetaminophen combination tablets for the treament of pain associated with osteoarthritis flare in an elderly patient population. J Am Geriatr Soc. 2004; 52:374–380.
26). Ruoff GE, Rosenthal N, Jordan D, Karim R, Kamin M. Tramadol/acetaminophen combination tablets for the treatment of chronic lower back pain: a multicenter, randomized, double-blind, placebo-controlled outpatient study. Clin Ther. 2003; 25:1123–1141.

27). Silverfield JC, Kamin M, Wu SC, Rosenthal N. Tramadol/acetaminophen combination tablets for the treatment of osteoarthritis flare pain: A multicenter, outpatient, randomized, double-blind, placebo-controlled, parallel-group, add-on study. Clin Ther. 2002; 24:282–297.

28). Smith AB, Ravikumar TS, Kamin M, Jordan D, Xiang J, Rosenthal N. Combination tramadol plus acetaminophen for postsurgical pain. Am J Surg. 2004; 187:521–527.

29). Mullican WS, Lacy JR. Tramadol/acetaminophen combination tablets and codeine/acetaminophen combination capsules for the management of chronic pain: A comparative trial. Clin Ther. 2001; 23:1429–1445.

30). Katz WA. Pharmacology and clinical experience with tramadol in osteoarthritis. Drugs. 1996; 52(S3):39–47.

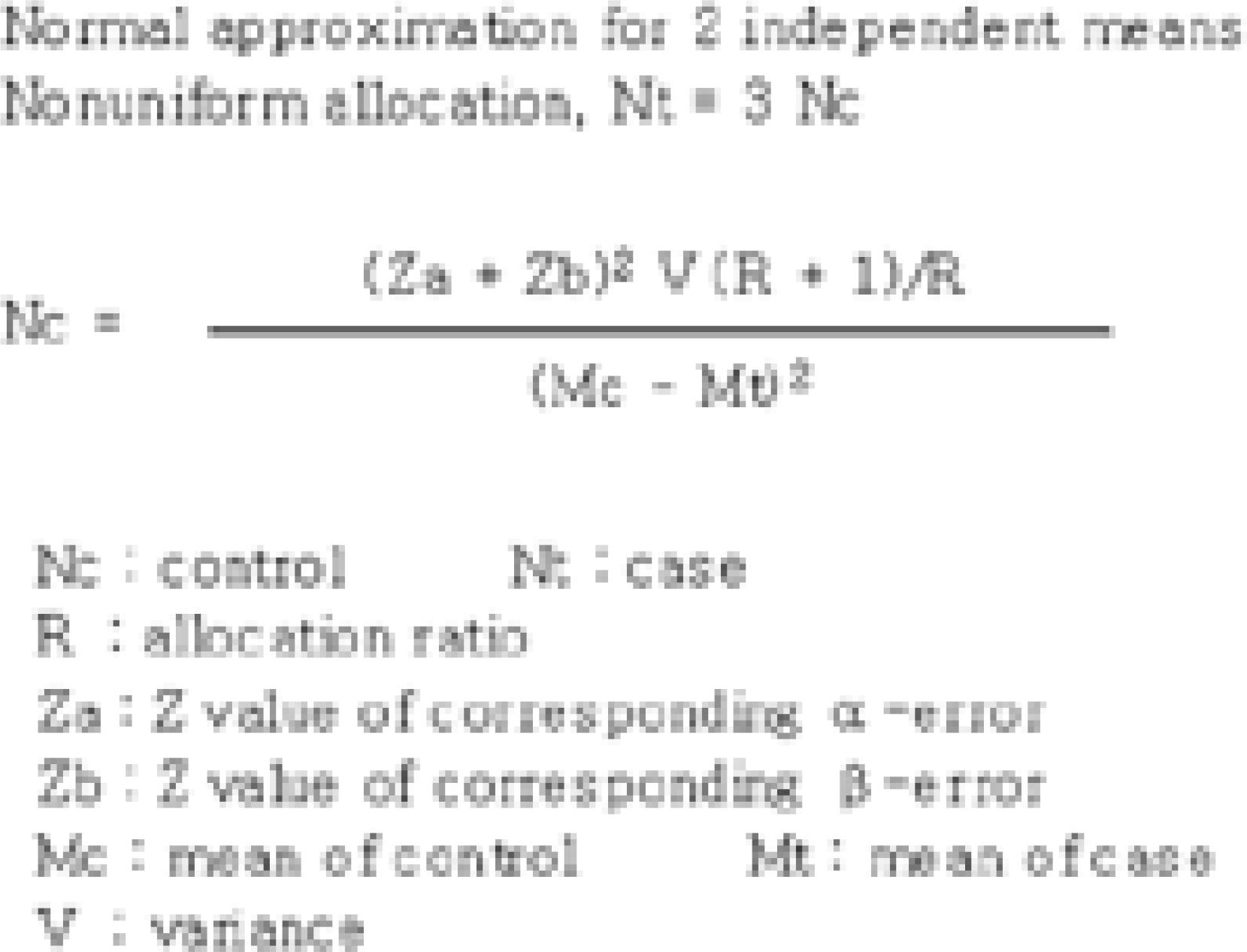
31). Donner A. Approaches to sample size estimation in the design of clinical trial-A review. Statistics in Medicine. 1984; 3:199–214.
32). Sunshine A, Olson NZ, Zighelboim I, De Castro A. Ketroprofen, acetaminophen plus oxycodone, and acetaminophen in the relief of postoperative pain. Clin Pharmacol Ther. 1993; 54:546–555.
33). Edwards JE, McQuay HJ, Moore RA. Combination analgesic efficacy: indivi-dual patient data meta-analysis of single-dose oral tramadol plus acetaminophen in acute postoperative pain. J Pain Symptom Manage. 2002; 3:121–130.
Go to : 
Figures and Tables%
Table 1.
Demographic and baseline characteristics
Table 2.
Study completion/withdrawal information
Table 3.
Baseline and final NRS of pain
| Ultracet | Placebo | p value* | |
|---|---|---|---|
| Baseline | 5.88±1.16 | 6.05±1.40 | 0.683 |
| Final | 2.54±2.41 | 4.10±2.20 | 0.008 |
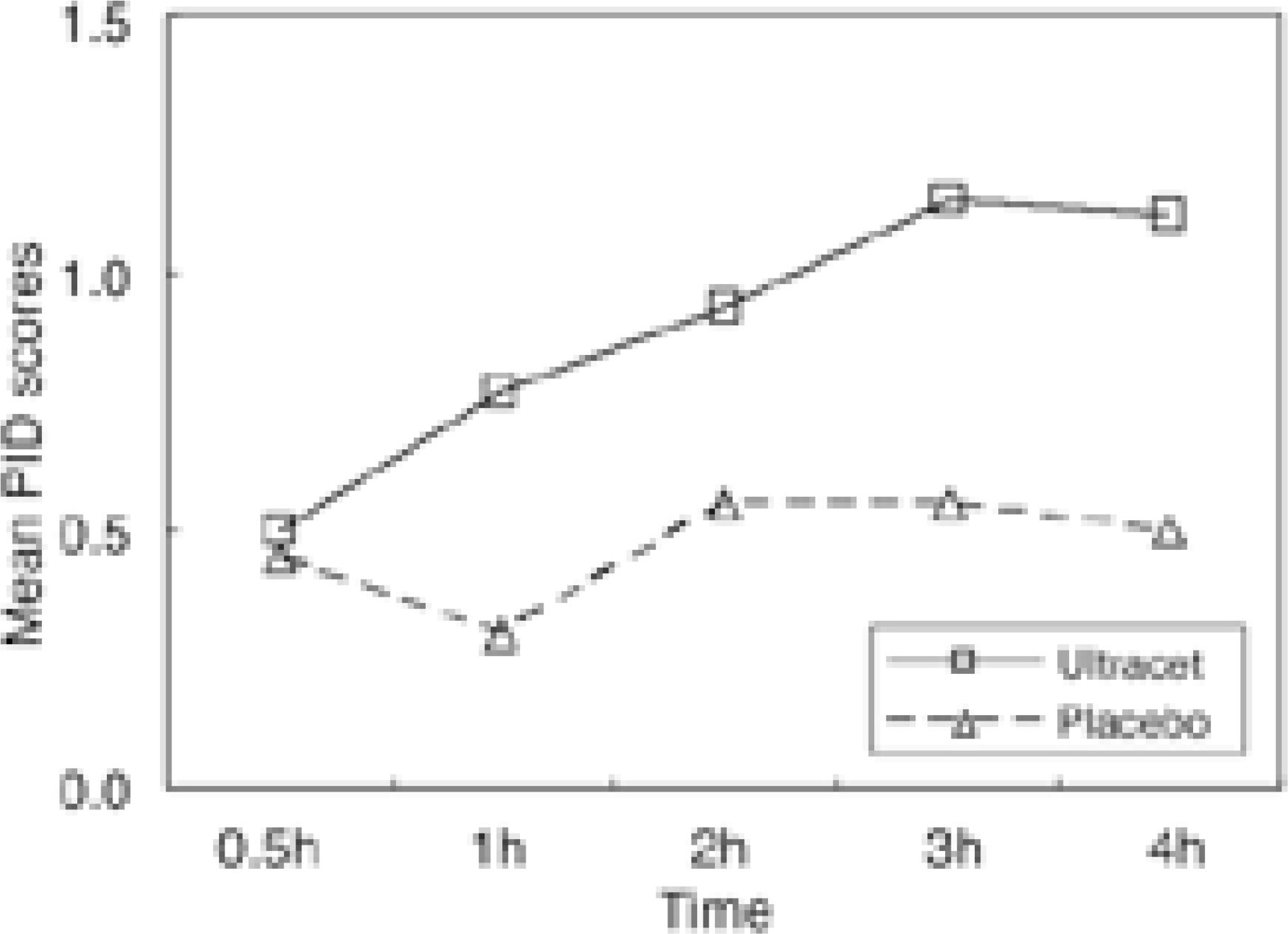
Table 4.
Mean pain intensity difference (PID) scores Unit : Mean±SD
| 30 min | 1 hour | 2 hour | 3 hour | 4 hour | |
|---|---|---|---|---|---|
| Ultracet | 0.50±0.63 | 0.77±0.74 | 0.93±0.83 | 1.14±0.88 | 1.11±0.89 |
| Placebo | 0.45±0.60 | 0.30±0.73 | 0.55±0.89 | 0.55±0.83 | 0.50±0.83 |
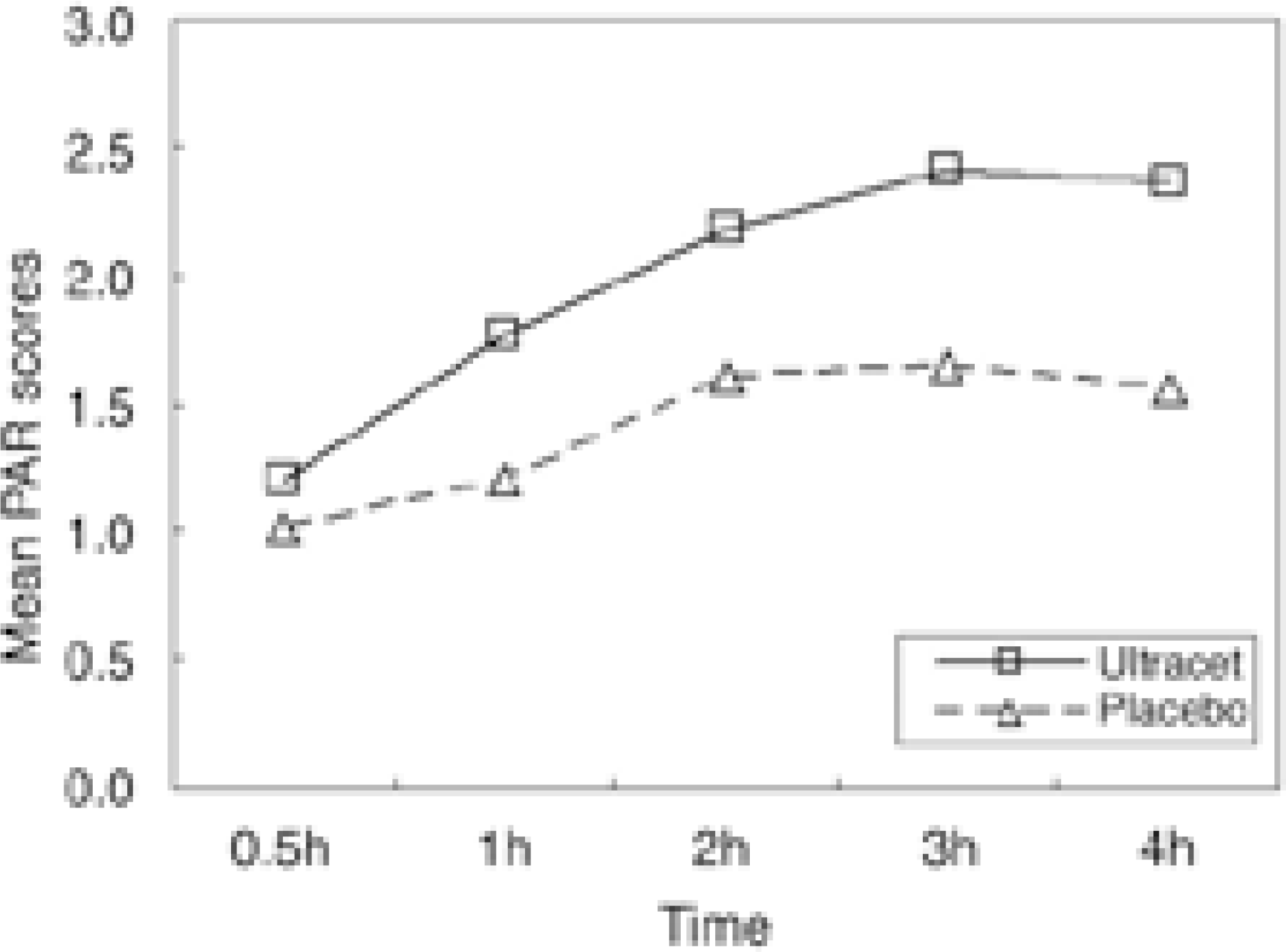
Table 5.
Mean pain relief(PAR) scores Unit : Mean±SD
| 30 min | 1 hour | 2 hour | 3 hour | 4 hour | |
|---|---|---|---|---|---|
| Ultracet | 1.20±1.10 | 1.77±1.27 | 42.18±1.38 | 2.41±1.49 | 2.36±1.51 |
| Placebo | 1.00±0.92 | 1.20±1.11 | 11.60±1.19 | 1.65±1.31 | 1.55±1.43 |
Table 6.
Results on the primary analgesic efficacy measures Unit : Mean±SD
| Ultracet | Placebo | p value* | |
|---|---|---|---|
| A. 0-4 Hours | |||
| SPID† | 3.81±2.9 | 51.98±3.02 | 0.028 |
| TOPAR† | 8.43±5.07 | 45.90±4.67 | 0.048 |
| B. 0-2 Hours | |||
| SPID | 1.56±1.38 | 0.93±1.48 | 0.093 |
| TOPAR | 3.66±2.36 | 2.70±2.11 | 0.112 |
| C. 2-4 Hours | |||
| SPID | 2.25±1.75 | 1.05±1.64 | 0.012 |
| TOPAR | 4.77±2.94 | 3.20±2.69 | 0.030 |
Table 7.
Time to onset of perceptible and meaningful pain relief
Table 8.
The adjusted risk ratio and their 95% confidence interval (CI) of perceptible and meaningful pain relief




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download