Abstract
Study Design
Objectives
Summary and Literature Review
Materials and Methods
Results
REFERENCES
Figures and Tables%
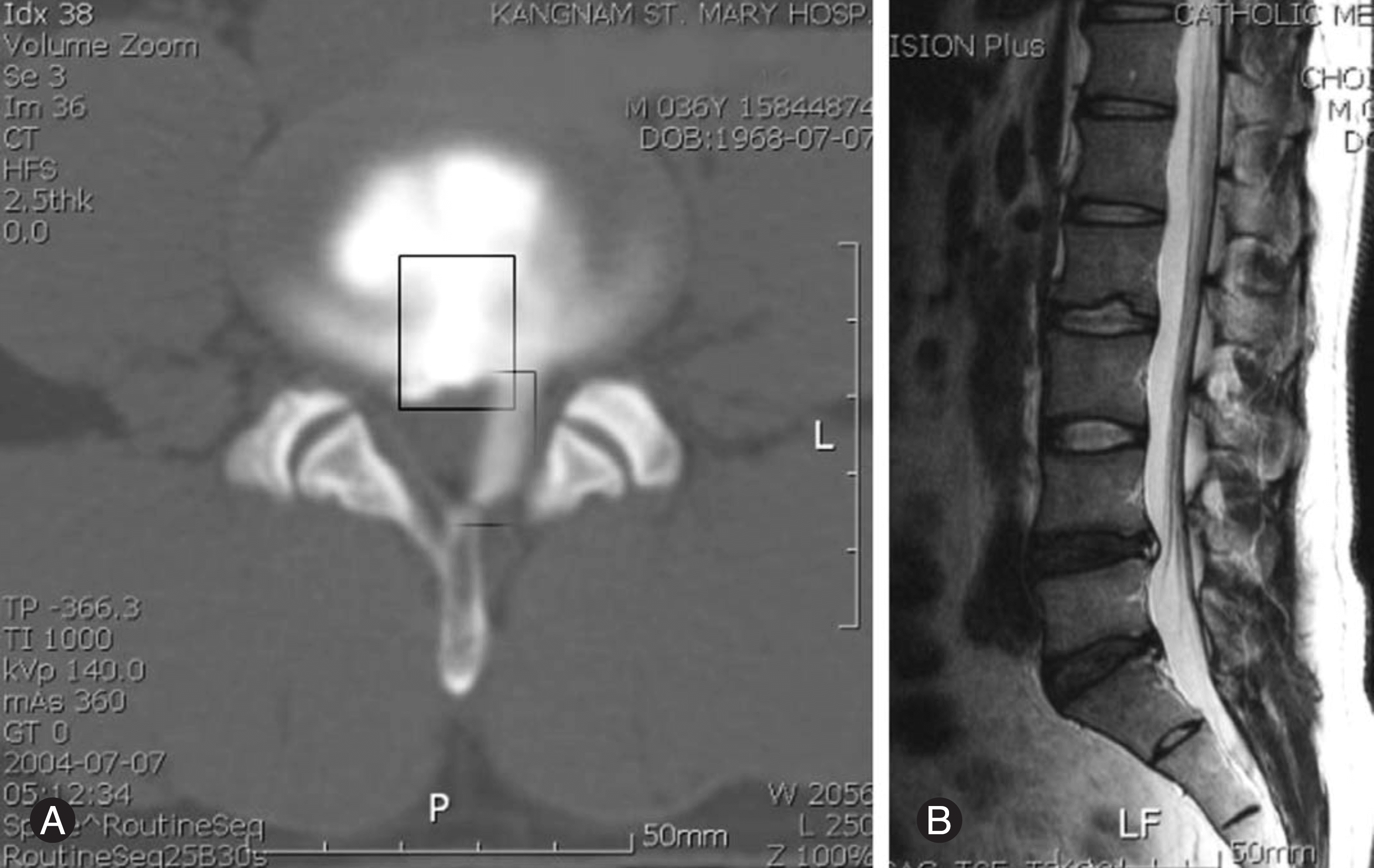 | Fig. 1.CT discography shows leakages of dye. Intervertebral disc specimens is obtained from the indicated area by rectangle (A). MRI of the same patient shows the degeneration of intervertebral disc of L4-5, L5-S1 and the hight intensity zone (B) |
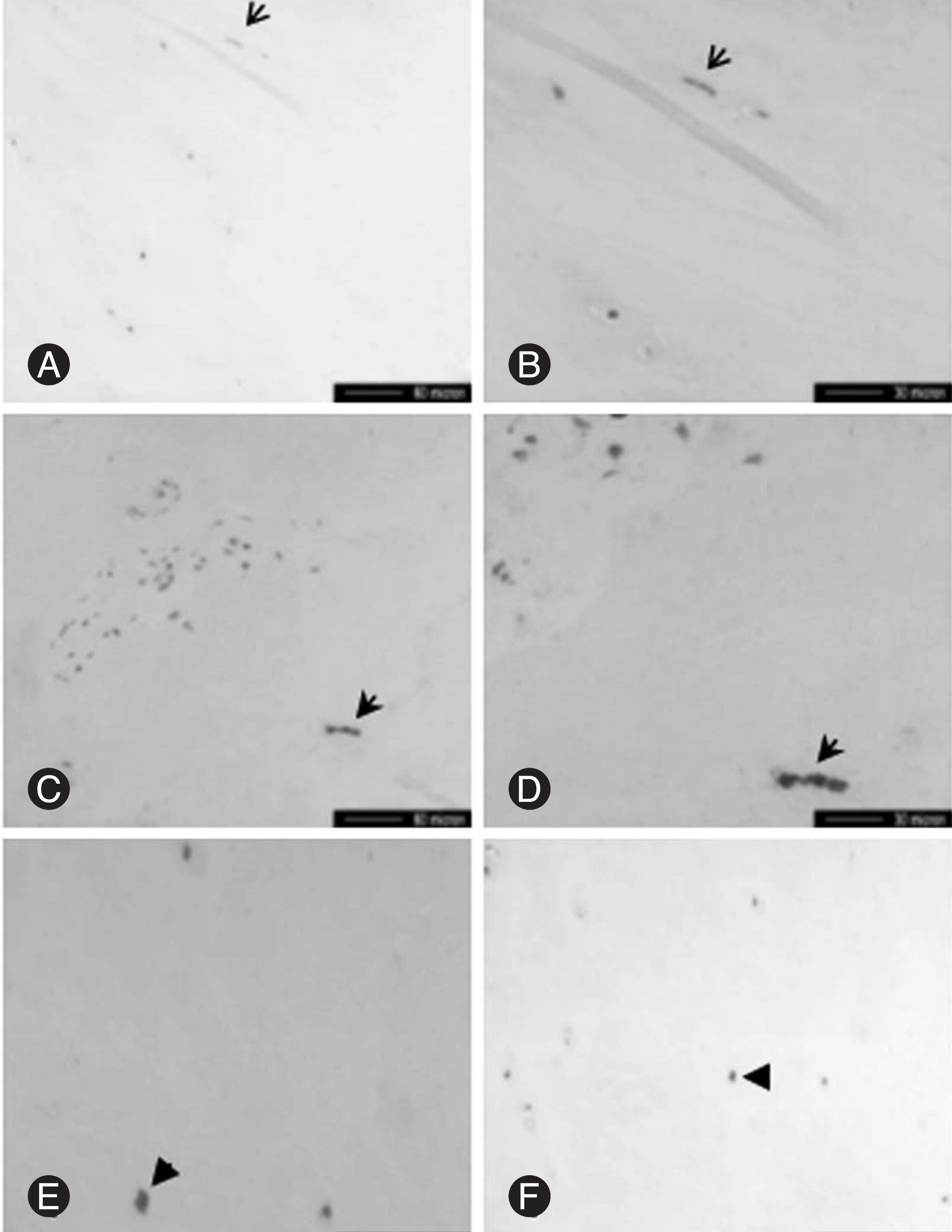 | Fig. 2.Photomicrographs showing evidence of innervation in internal disc disruption. SP-immunoreactive nerve fiber is present in outer one third of the annulus fibrosus (arrows in A and B), and in the nucleus pulposus, with longitudinal (open arrows in C and D) and cross (arrows in E and F) section. (SP: substance P). |
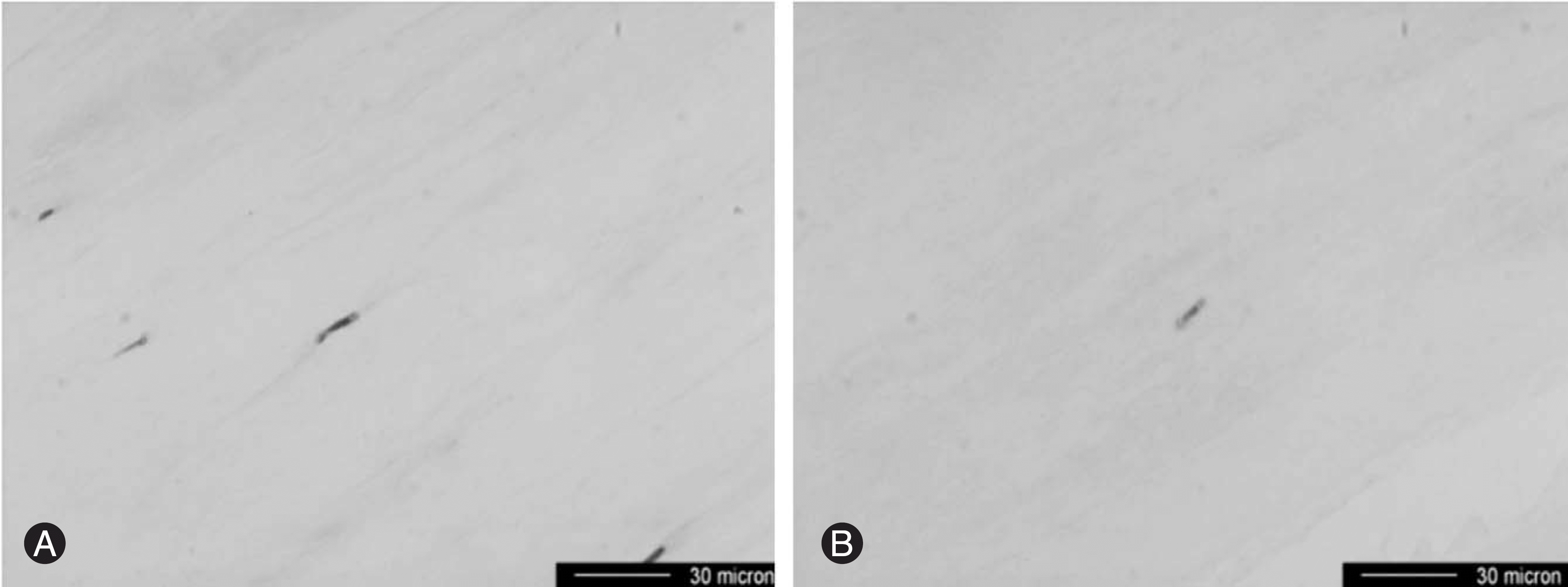 | Fig. 3.(A, B) GAP43-immunoreactive nerve fiber (arrow) is present in the annulus fibrosus of IDD (A) and of HNP (B). |
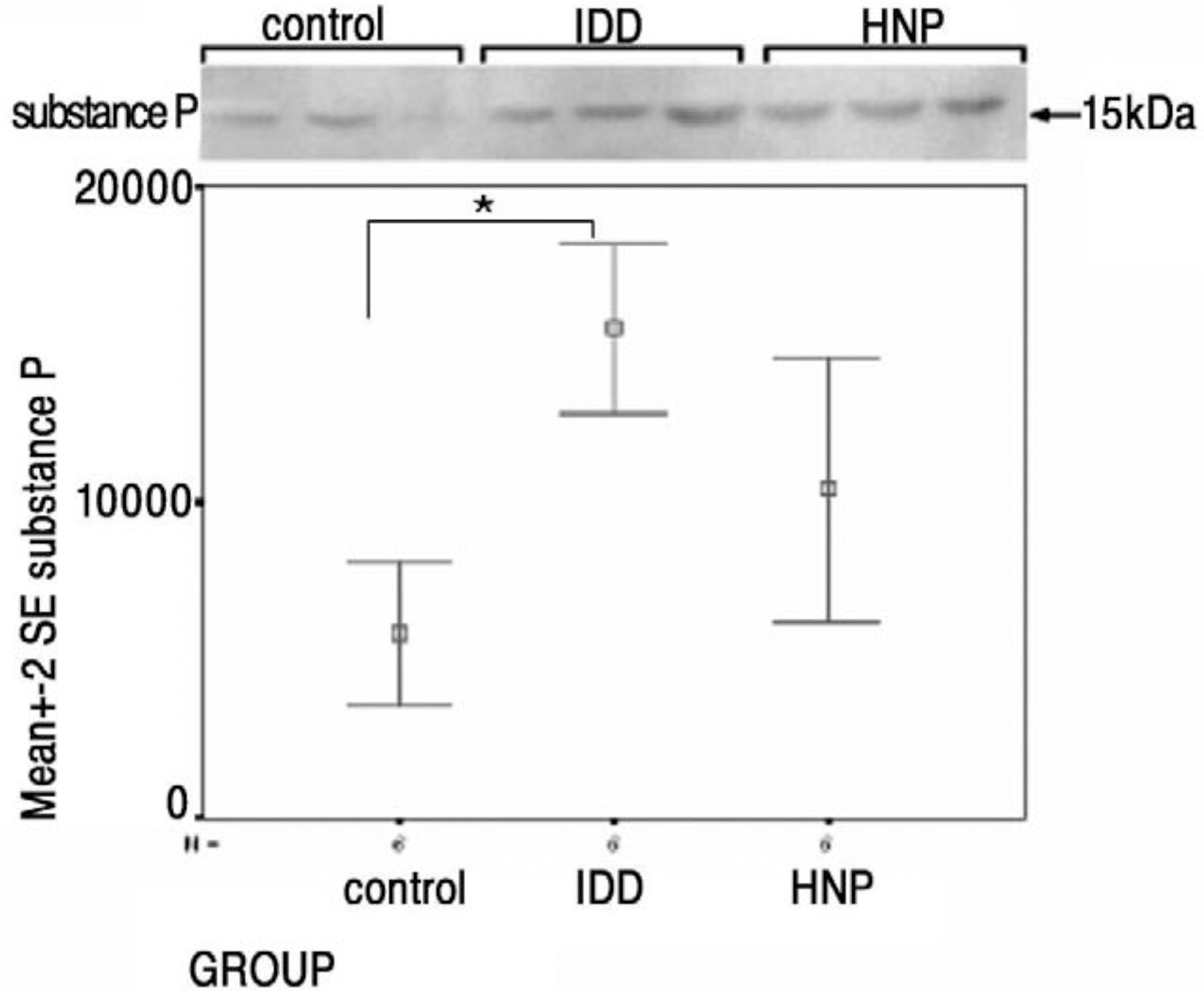 | Fig. 4.Westen blots and statistical analysis. Statistically significantly more expression of substance P in IDD than control group (p IDD-control=0.002) (one-way ANOVA; SPSS 11.0 for windows). |
Table 1.
Table 2.
| No.* | Concordant pain | MRI grade† | HIZ† | Decreased disc height | End plate abnormality (Modic type**) | Facet osteoarthritis+ (grade) | Discography++ |
|---|---|---|---|---|---|---|---|
| 1 | + | 5 | - | + | I | 2 | V |
| 2 | + | 5 | + | + | - | 1 | V |
| 3 | + | 4 | - | + | - | 2 | V |
| 4 | + | 5 | + | - | I | 2 | V |
| 5 | + | 4 | + | + | I | 2 | V |
| 6 | + | 5 | + | + | III | 3 | V |
| 7 | + | 5 | + | - | III | 1 | V |
| 8 | + | 5 | + | + | I | 1 | V |
| 9 | + | 3 | - | + | III | 1 | V |
| 10 | + | 5 | - | + | II | 1 | V |
† : grade 3. mild degeneration, blurred differentiation of the nucleus pulposus from the annulus, slightly decreased signal of the nucleus pulposus with minor irregularities; grade 4, moderate disc degeneration, blurred differentiation of the nucleus pulposus from the annulus, moderately decreased signal of the nucleus pulposus, with hypointense zones; grade 5, severe degeneration, a loss of differentiation of the nucleus pulposus from the annulus, hypointense signal of the nucleus pulposus with or without a hori-zontal hyperintense band.
† : focal zone of high signal intensity within the posterior aspect of the annulus fibrosus, without focal protrusion or extrusion of the disc.
∗ : type I, low signal intensity of T1-weighted images and high signal intensity on T2-weighted images; type II, high signal intensity on both images; type III, low signal intensity on both images.
+ : grade 1, mild degenerative disease(indicated by narrowing of the facet joint space, small osteophytes, and/or mild hypertrophy of the articular process); grade 2, moderate degenerative disease(indicated by narrowing of the facet joint space, moderate osteophytes, moderate hypertrophy of the articular process, and/or mild subarticular erosions); grade 3, severe degenerative disease(indicated by narrowing of the facet joint space, severe osteophytes, severe hypertrophy oh the articular process, severe subarticular erosions, and/or subchondral cysts).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download