Abstract
Study Design
Objectives
To establish a daughter cell line, B2A1, from B2 cells through the limiting dilution method, and to determine if the cells have the characteristics of neural stem cell (NSCs) using immunocytochemistry and RT-PCR methods.
Summary and Literature Review
In the development of NSCs, differentiated organ or tissue-derived multipotent stem cells have attracted considerable interest because of the lack of ethical issues. Previously, a glial precursor cell line (B2 cells) was generated from the primary cultures of oligodendrocytes/ astrocytes in an adult BALB/c mouse brain. These cells exhibited the cell-type specific markers for immature neuroectodermal cells, astrocytes, and oligodendrocytes in serum-contained media.
Materials and Methods
The primary cultures of oligodendrocytes/astrocytes were established from the whole brains of 12 to 16-week-old BALB/c mice from either gender. After 6 months with 25 serial passages, the culture consisted of a morphologi-cally homogeneous cell population, which was designated as B2 cells. A subclone, B2A1, was isolated from B2 cells through two consecutive limiting dilutions.
Results
More than 90% of B2A1 cells showed immunopositivity for nestin, a specific marker for NSC. The cells also showed immunopositivity for the neuronal, astrocytic and oligodendroglial markers. These cells expressed the genotypic mRNA messages for both neural progenitor cells and differentiated neuronoglial cells. These positive immunocytochemical reactions and mRNA messages for neuronoglial cells varied according to the extrinsic growth factors used. However, the treatment of extrinsic growth factors did not produce any significant differences in the nestin-immunopositive cells.
Go to : 
REFERENCES
1). Bartlett PF, Brooker GJ, Faux CH, et al. Regulation of neural stem cell differentiation in the forebrain. Immun Cell Biol. 1998; 76:414–418.

2). Brustle O, Spiro AC, Karram K, Choudhary K, Okabe S, McKay RD. In vitro-generated neural precursors par-ticipate in mammalian brain development. Proc Natl Acad Sci USA. 1997; 94:14809–14814.

3). Flax JD, Aurora S, Yang C. Engraftable human neural stem cells respond to developmental cues, replace neurons, and express foreign genes. Nat Biotechnol. 1998; 16:1033–1039.
4). Thomoson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998; 282:1145–1147.

5). Shamblott MJ, Axelman J, Wang S, et al. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc Natl Acad Sci USA. 1998; 95:13726–13731.

6). Davis AA, Temple S. A self-renewing multipotential stem cell in embryonic rat cerebral cortex. Nature. 1994; 372:263–266.

8). Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996; 16:2027–2033.

9). Kukekov VG, Laywell ED, Suslov O, et al. Multipotent stem/progenitor cells with similar properties arise from two neurogenic regions of adult human brain. Exp Neurol. 1999; 156:333–344.

10). Anderson DJ. Stem cells and pattern formation in the nervous system: the possible versus the actual. Neuron. 2001; 30:19–35.
11). Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001; 21:6718–6731.

12). Satoh J, Tabira T, Kim SU. Rapidly proliferating glial cells isolated from adult mouse brain have a differentiative capacity in response to cyclic AMP. Neurosci Res. 1994; 20:175–184.

13). Satoh J, Kim SU, Kastrukoff LF, Takei F. Expression and induction of intercellular adhesion molecules (ICAMs) and major histocompatibility complex (MHC) antigens on cultured murine oligodendrocytes and astrocytes. J Neurosci Res. 1991; 29:1–12.

14). Rey JA, Bello MJ, Decampos JM, Kusak ME, Moreno S. Cytogenetic analysis in human neurinomas. Cancer Genet Cytogenet. 1987; 28:187–188.

15). Satoh J, Gallyas F Jr, Endoh M, Yamamura T, Kun-ishita T, Tabira T. Coexistence of cholinergic, cate-cholaminergic, serotonergic, and glutamatergic neurotransmitter markers in mouse clonal hybrid neurons derived from the septal region. J Neurosci Res. 1992; 32:127–137.

16). Kim SU, Stern J, Kim MW, Pleasure DE. Culture of purified rat astrocytes in serum-free medium supplement-ed with mitogen. Brain Res. 1983; 274:79–86.

17). Ranscht B, Clapshaw PA, Price J, Noble M, Seifert W. Development of oligodendrocytes and Schwann cells studied with a monoclonal antibody against galactocerebroside. Proc Natl Acad Sci USA. 1982; 79:2709–2713.

18). Nagai A, Suzuki Y, Baek SY, et al. Generation and Characterization of Human Hybrid Neurons Produced between Embryonic CNS Neurons and Neuroblastoma Cells. Neu-robiol Dis. 2002; 11:184–198.

19). Cai J, Wu Y, Mirua T, et al. Properties of a fetal multipotent neural stem cell (NEP cell). Dev Biol. 2002; 251:221–240.

20). Goodell MA, Brose K, Paradis G, Conner AS, Mulli-gan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996; 183:1797–1806.

21). Bjornson CR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999; 283:534–537.

22). Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999; 284:143–147.

23). Dahl D, Rueger DC, Bignami A, Weber K, Osborn M. Vimentin, the 57 000 molecular weight protein of fibrob-last filaments, is the major cytoskeletal component in immature glia. Eur J Cell Biol. 1981; 24:191–196.
24). Bignami A, Raju T, Dahl D. Localization of vimentin, the nonspecific intermediate filament protein, in embryonal glia and in early differentiating neurons. In vivo and in vitro immunofluorescence study of the rat embryo with vimentin and neurofilament antisera. Dev Biol. 1982; 91:286–295.
25). Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990; 60:585–595.

26). Gates MA, Thomas LB, Howard EM, et al. Cell and molecular analysis of the developing and adult mouse sub-ventricular zone of the cerebral hemispheres. J Comp Neurol. 1995; 361:249–266.

27). Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res Dev Brain Res. 1995; 84:109–129.

28). Zhong W, Jiang MM, Weinmaster G, Jan LY, Jan YN. Differential expression of mammalian Numb, Numblike and Notch1 suggests distinct roles during mouse cortical neurogenesis. Development. 1997; 124:1887–1897.

29). Josephson R, Muller T, Pickel J, et al. POU transcription factors control expression of CNS stem cell-specific genes. Development. 1998; 125:3087–3100.

30). Kageyama R, Ohtsuka T. The Notch-Hes pathway in mammalian neural development. Cell Res. 1999; 9:179–188.

31). Yang X, Handler M, Shen J. Role of presenilin-1 in murine neural development. Ann N Y Acad Sci. 2000; 920:165–170.

32). Kishi M, Mizuseki K, Sasai N, et al. Requirement of Sox2-mediated signaling for differentiation of early Xeno-pus neuroectoderm. Development. 2000; 127:791–800.

33). Johe KK, Hazel TG, Muller T, Dugich-Djordjevic MM, McKay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996; 10:3129–3140.

34). Andrae J, Hansson I, Afink GB, Nister M. Platelet-derived growth factor receptor-alpha in ventricular zone cells and in developing neurons. Mol Cell Neurosci. 2001; 17:1001–1013.
35). Martinez-Serrano A, Lundberg C, Horellou P, et al. CNS-derived neural progenitor cells for gene transfer of nerve growth factor to the adult rat brain: complete res-cue of axotomized cholinergic neurons after transplantation into the septum. J Neurosci. 1995; 15:5668–5680.

Go to : 
Figures and Tables%
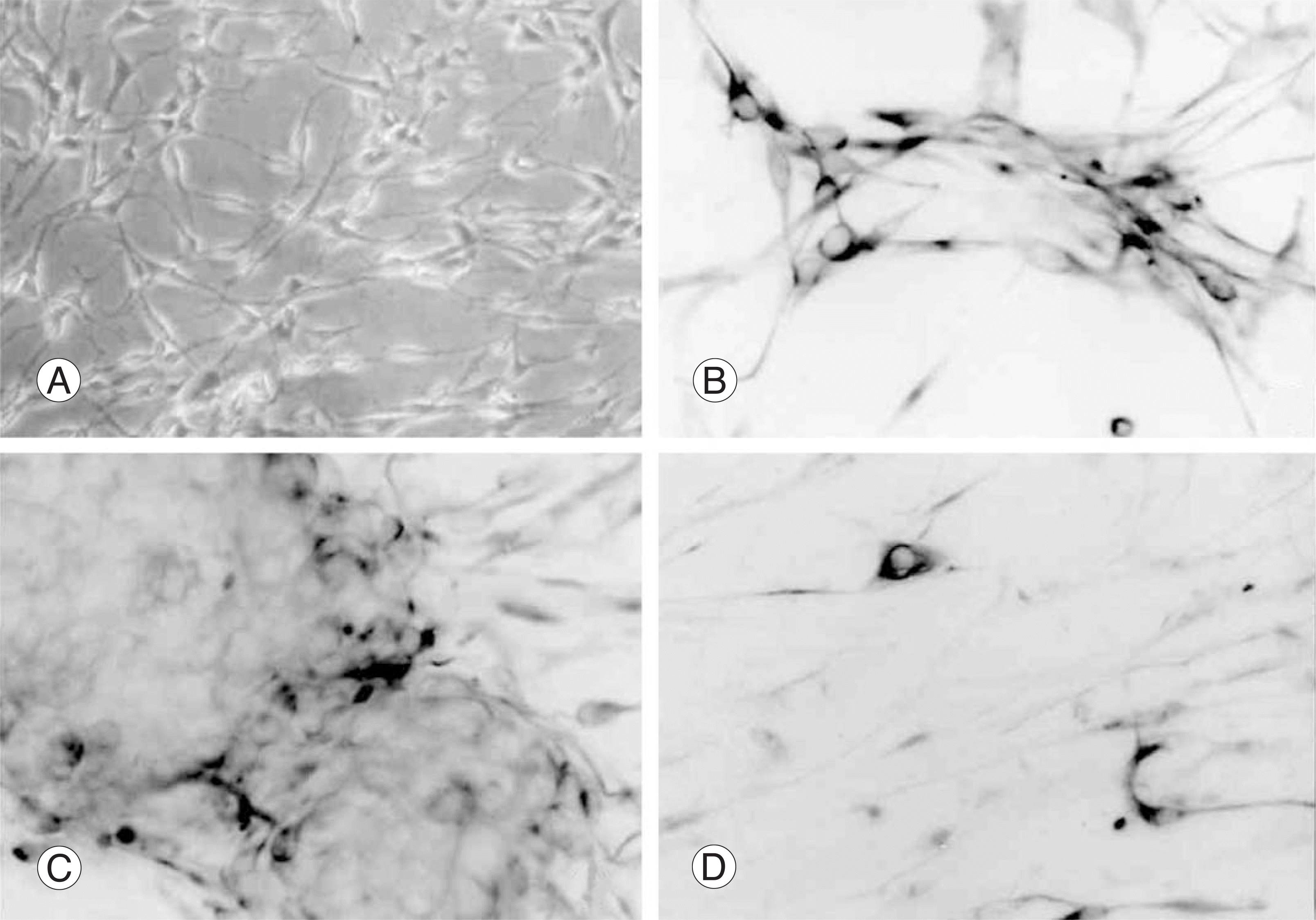 | Fig. 1.Cytologic characteristics of B2A1 cells disclosed bi- or tripolar cells (A) phase contrast microscopy (B) and show immunopositivities for nestin (C) β tubulin III, (D) and GFAP. |
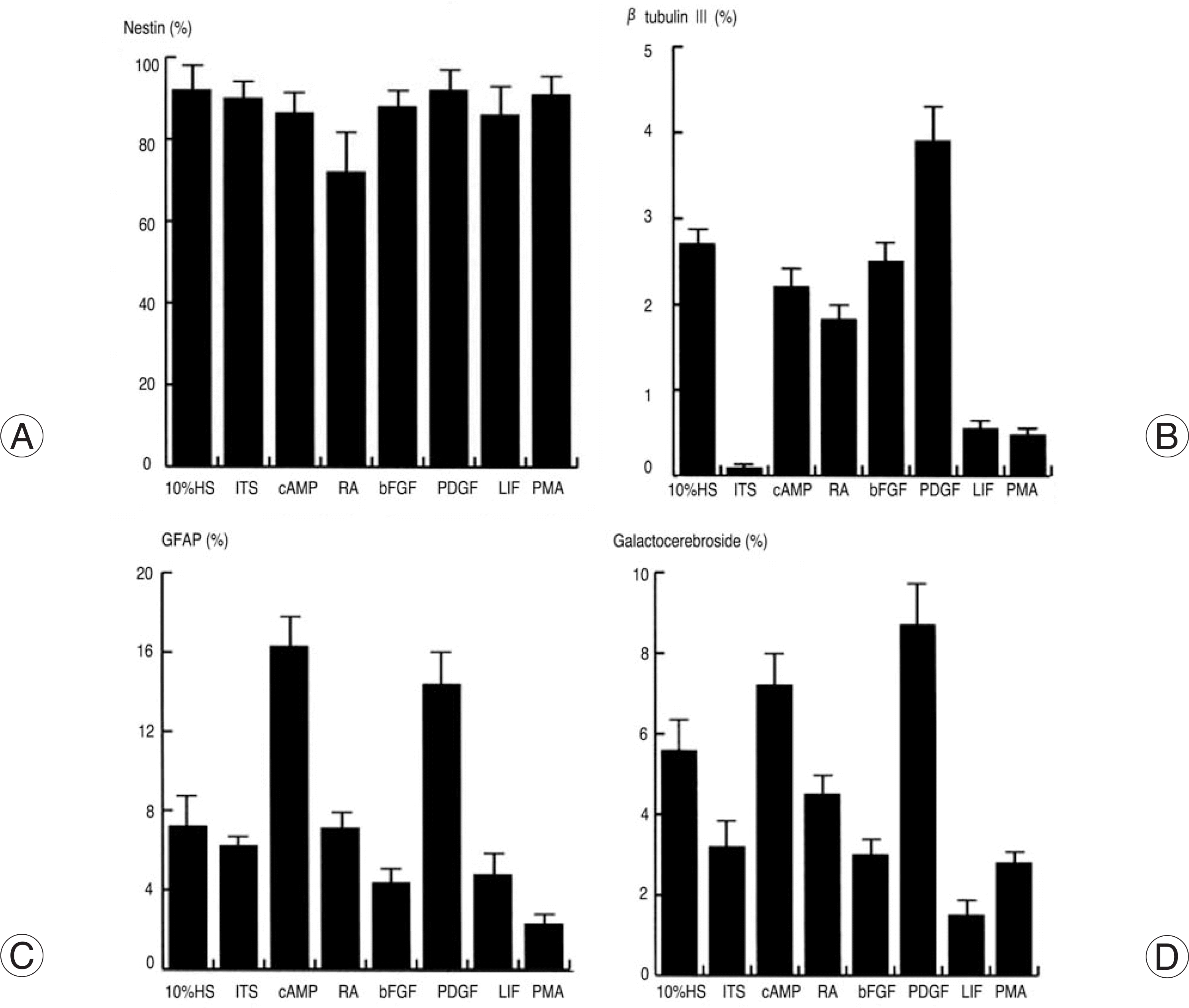 | Fig. 2.Multipotential differentiation of B2A1 cells by treatment of growth factors. Most of all cells were immunopositive for nestin in any culture condition (A). Immunopositive cells for βtubulin III significantly increased in PDGF-contained media (B), while GFAP (C) and galactocerebroside (D) positive cells markedly increased in c-AMP or PDGF-contained media. |
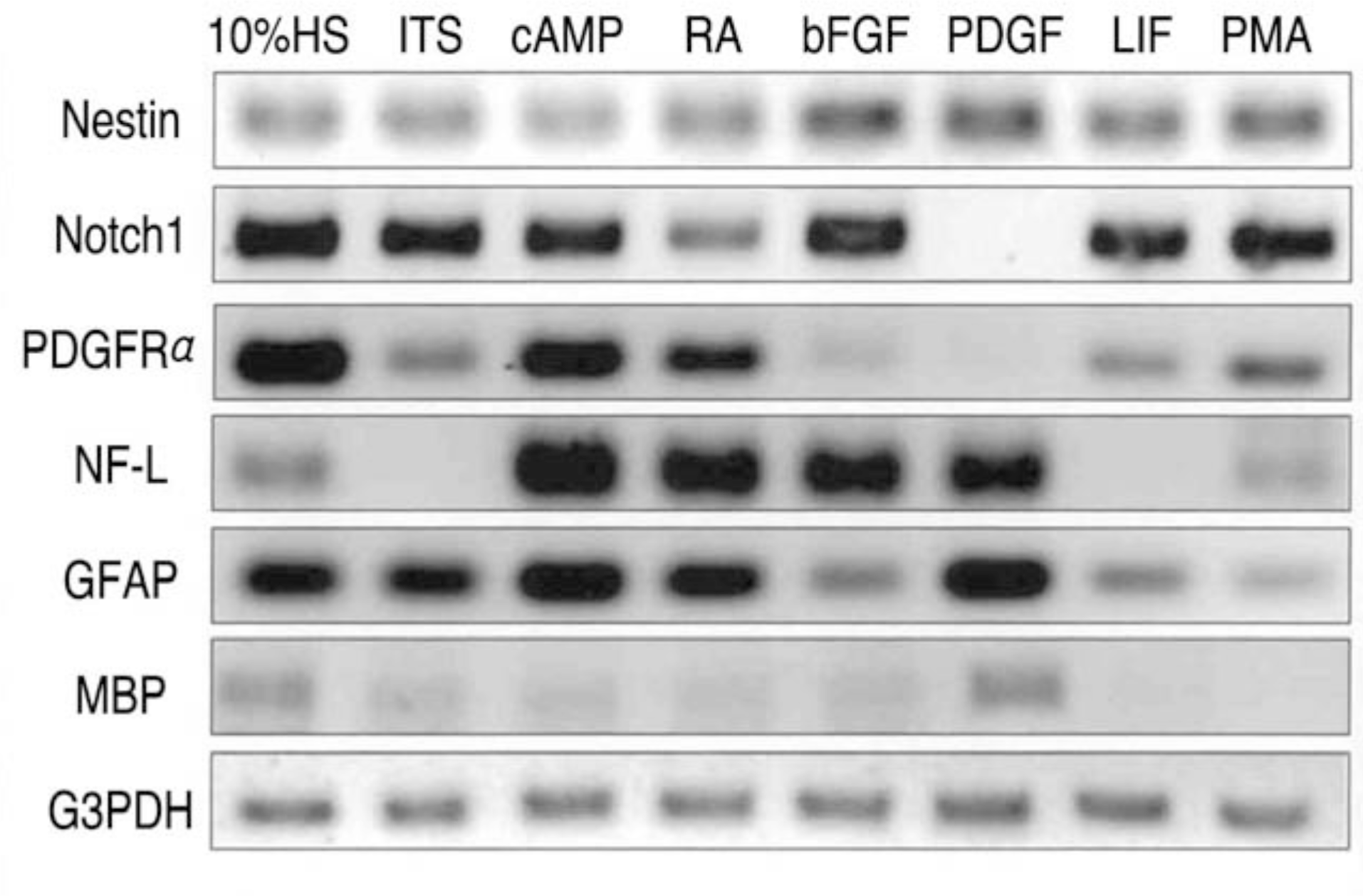 | Fig. 3.Gene expression of cell type specific markers studied by RT-PCR in B2A1 cells. The cells expressed both neural progenitor cell markers (nestin, Notch1, PDGFα) and differentiated cell markers for neurons (NF-L), astrocytes (GFAP), and oligodendrocytes (MBP). The expression levels were variable related to addition of growth factors. |
Table 1.
Cell type specific immunocytochemical markers
Table 2.
Sequence of PCR primers




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download