Abstract
Objectives
To evaluate the mRNA expressions of matrix components, and analyze the cellular proliferation and proteoglycan synthesis of human intervertebral disc cells in response to dexamethasone and TGF- β1
Summary of Literature Review
Corticosteroids are responsible for the regulation of a diverse range of biological processes through modulation of the expression of target genes. The direct injection of methylprednisolone to the intervertebral disc (IVD) has been shown to cause degeneration and calcification of the disc in rabbits. Systemic administration of hydrocortisone induced degeneration of notochordal cells, which accelerated the aging process of the disc in mice. Transforming growth factor beta- 1(TGF- β1) is known as a potent agent for the proliferation, differentiation and matrix synthesis of IVD.
Materials and Methods
IVD cells were isolated from ten patients, and subsequently cultured. Various doses of dexamethasone (DEX) and/or TGF- β1 were administered to the IVD cultures. DNA and proteoglycan syntheses were measured by the incorporation of [3H]- thymidine and [35S]- sulfate, respectively. RT-PCRs were performed for the expressions of aggrecan, collagen types I and II, and osteocalcin mRNA.
Res ults
Cultures with DEX showed increased cellular proliferation and decreased proteoglycan synthesis (p<0.05). TGF- β1 potentiated the proliferative effect of DEX, but failed to stimulate proteoglycan synthesis in the cultures containing DEX. There were no recognizable changes in the mRNA expressions of aggrecan, collagen types I and II, and osteocalcin in response to DEX and TGF- β1.
Conclusions
DEX demonstrated a proliferative effect on human IVD cells, with the combination of DEX and TGF- β1 showing potentiation of the proliferative effect, while at high doses(100 and 1000nM, the DEX was shown to down- regulate the proteoglycan synthesis. Caution should be exercised in the use of corticosteroid in the therapeutic approaches for the treatment of disc disease or in the regenerative matrix of the IVD.
Go to : 
REFERENCES
1). Behrend F, Kemppainen RJ. Glucocorticoid therapy, pharmacology, indications, and complications. Vet Clin North Am Small Anim Pract. 1997; 27:187–213.
2). Gotti E, Perico N, Perna A, et al. Renal transplantation: can we reduce calcineurin inhibitor/stop steroids? Evidence based on protocol biopsy findings. J Am Soc Nephrol. 2003; 14:755–766.

3). Parameswaran K, Leigh R, O'Byrne PM, et al. Clinical models to compare the safety and efficacy of inhaled corticosteroids in patients with asthma. Can Respir J. 2003; 10:27–34.

4). Young AL, Rao SK, Cheng LL, Wong AK, Leung AT, Lam DS. Combined intravenous pulse methylprednisolone and oral cyclosporine A in the treatment of corneal graft rejection: 5 year experience, Eye. 2002; 16:304–308.
5). Grassi W, Farina A, Filippucci E, Cervini C. I ntralesional therapy in carpal tunnel syndrome: a sonographic-guided approach. Clin Exp Rheumatol. 2002; 20:73–76.
6). Raynauld JP, Buckland-Wright C, Ward R, et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, doubleblind, placebo-controlled trial. Arthritis Rheum. 2003; 48:370–377.

7). Pelletier JP, Mineau F, Raynauld JP, Woessner JR, Gunjasmith Z, Martel-Pelletier J. Intraarticular injection with methylpredisolone acetate reduce osteoarthritic lesion in parallel with chondrocyte stromelisin synthesis in experimental osteoarthritis. Arthritis Rheuma. 1994; 37:414–423.
8). Grigoriadis AE, Heersche JN, Aubin JE. Differentiation of muscle, fat, cartilage and bone from progenitor cells present in a bone marrow derived clonal cell population, effect of dexamethasone. J Cell Biol. 1988; 106:2139–2151.
9). Takano T, Takigawa M, Suzuki F. Stimulation by glu -cocorticoids of the differentiated phenotype of chondrocytes and the proliferation of rabbit costal chondrocytes in culture. J Biochem. 1985; 97:1093–1100.
10). Silbermann M, Maor G. Receptor mediated glucocorticoid inhibition of cell proliferation in mouse growth cartilage in vivo. Acta Endocriolo. 1985; 108:343–350.
11). Moskowitz RW, Davis W, Sammarco J, Mast W, Chase SW. Experimentally induced corticosteroid arthropathy. Arthitis Rheuma. 1970; 13:263–277.

12). Salter RB, Gross A, Hall JH. Hydrocortisone arthropathy, an experimental investigation. Can Med J. 1967; 97:374–377.
13). Chunekamrai S, Knook LP, Lust G, Maylin GA. Changes in articular cartilage after intraarticular injections of methylprednisolone acetate into equine joints. Am J Vet Res. 1989; 50:1733–1741.
14). Trotter GW, McIlwraith CW, Yovich JV, et al. Effects of intraarticular administeration of methylpredinisolone acetate on normal equine articular catilage. Am J Vet Res. 1991; 52:83–87.
15). Tressler RH and Salmon WD. Glucocorticoid inhibition of sulfate incorporation by cartilage of normal rat. Endocrinology. 1975; 96:898–902.
16). Higuchi M, Abe K. Ultrastructure of the nucleus pulposus in the intervertebral disc after systemic administration of hydrocortisone in mice. Spine. 1985; 10:638–643.

17). Aoki M, Kato F, Minmatsu K, Iwata H. His tolog i c changes in intervertebral disc after intradiscal injections of methylprednisolone acatate in rabbits. Spine. 1997; 22:127–131.
18). Minamida A, Tamaki T, Hashizume H, Yoshida M, Kawakami M, Hayashi N. Effects of steroid and lipopolysaccharide on spontaneous resorption of herniated intervertebral disc, an experimental study in the rabbit. Spine. 1998; 23:870–876.
19). Gruber HE, Fisher EC, Desani B, Stasky AA, Hoelsch-er G, Hanley EN. Human intervertebral disc cells from the annulus: three dimensional culture in agarose or alginate and responsiveness to TGF-b1. Exp Cell Res. 1997; 235:13–21.
20). Thompson JP, Oegema TR, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991; 16:253–260.

21). Moon SH, Nishida K, Gilbertson LG, Hall RA, Robbins PD, Kang JD. Cocktail therapeutic gene transfer to human intervertebral disc cells cultured in three-dimensional alginate beads. Proceedings of North American Spine Society, New Orleans. 2000.
22). Nishida K, Kang JD, Gilbertson LG, et al. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: An in vivo study of adenovirus-mediated transfer of the human transforming growth factor β1 encoding gene. Spine. 1999; 24:2419–2425.
23). Cutroneo KR. Relationship between glucocorticoid-medi -ated early decrease of protein synthesis and the steady state decrease of glucocorticoid receptor and TGF-b acti -vator protein. Int J Biochem Cell Biol. 2002; 34:194–203.
24). Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell. 1998; 93:487–490.

25). Song CZ, Tian X, Gelehert TD. Glucocorticoid receptor inhibits transforming growth factor beta signalling by directly targeting the transcriptional activation function of Smad3. Pro Natl Acad Sci. 1999; 96:1776–1781.
26). Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997; 4(390):(6659):. 465–471.
27). Massague J, Blain SW, Lo RS. TGF-β signaling in growth control, cancer, and heritable disorders. Cell. 2000; 103:295–309.

28). Guller S, Wonzniak R, Kong L, Lockwood CJ. Opposing actions of transforming growth factor-beta and glu -cocorticoids in the regulation of fibronectin expression in the human placenta. J Clin Endocrinol Metabol. 80:3272–3278.
29). Slavin J, Unemori E, Hunt T Amento E. Transforming growth factor beta (TGF-beta) and dexamethasone have direct opposing effects on collagen metabolism in low pas - sage human dermal fibroblasts in vitro. Growth factor. 1994; 11:205–213.
30). Guan Y, Woo PL, Rubenstein NM, Firestone GL. Transforming growth factor alpha abrogates the glucocorticoid stimulation of tight junction formation and reverse the steroid induced downregulation of fascin in rat mam -mary epithelial tumor cells by a Ras-dependent pathway. Exp Cell Res. 2002; 273:1–11.
31). Hardingham TE, Adams P. A. method for the determi -nation of hyaluronate in the presence of other gly -cosaminoglycans and its application to human intervertebral discs. Biochem J. 1976; 159:143–147.
32). Lipson SJ, Muir H. Proteoglycans in experimental intervertebral disc degeneration. Spine. 1981; 6:194–210.

33). Melrose J, Ghosh P, Taylor TKF, et al. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibrosus. J Orthop Res. 1992; 10:665–676.

34). Pearce RH, Grimmer BJ, Adams ME. De ge nerat ion and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987; 5:198–205.
35). Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995; 20:1307–1314.

36). Ganey TM, Meisel HJ. A potential role for cell-based therapeutics in the treatment of intervertebral disc herniation. Eur Spine J. 2002; 2:S206–214.

38). Gruber HE, Johnson TL, Leslie K, et al. Autologo us intervertebral disc cell implantation: a model using Psam -momys obesus, the sand rat. Spine. 2002; 27:1626–1633.
39). Sato M, Asazuma T, Ishihara M, et al. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine. 2003; 28:548–553.

40). Moon SH, Gilbertson LG, Nishida K, et al. Hum an intervertebral disc cells are genetically modifiable by ade -novirus-mediated gene transfer. Spine. 2000; 25:2573–2579.
41). Nishida K, Kang JD, Suh J-K, Robbins PD, Evans CH, Gilbertson LG. Adenovirus-mediated gene transfer to nucleus pulposus cells: Implication for the treatment of intervertebral disc degeneration. Spine. 1998; 3:2437–2443.
42). Ito S, Usui H, Maruyama K, Muro T. Roentgenographic evaluation of ossification and calcification of the lumbar spinal canal after intradiscal betamethasone injection. J Spinal Disord. 2001; 14:434–438.

43). Simmons JW, McMillin JN, Emery SF, Kimmich SJ. Intradiscal steroids. A prospective double-blind clinical trial. Spine. 1992; 17:S172–175.
Go to : 
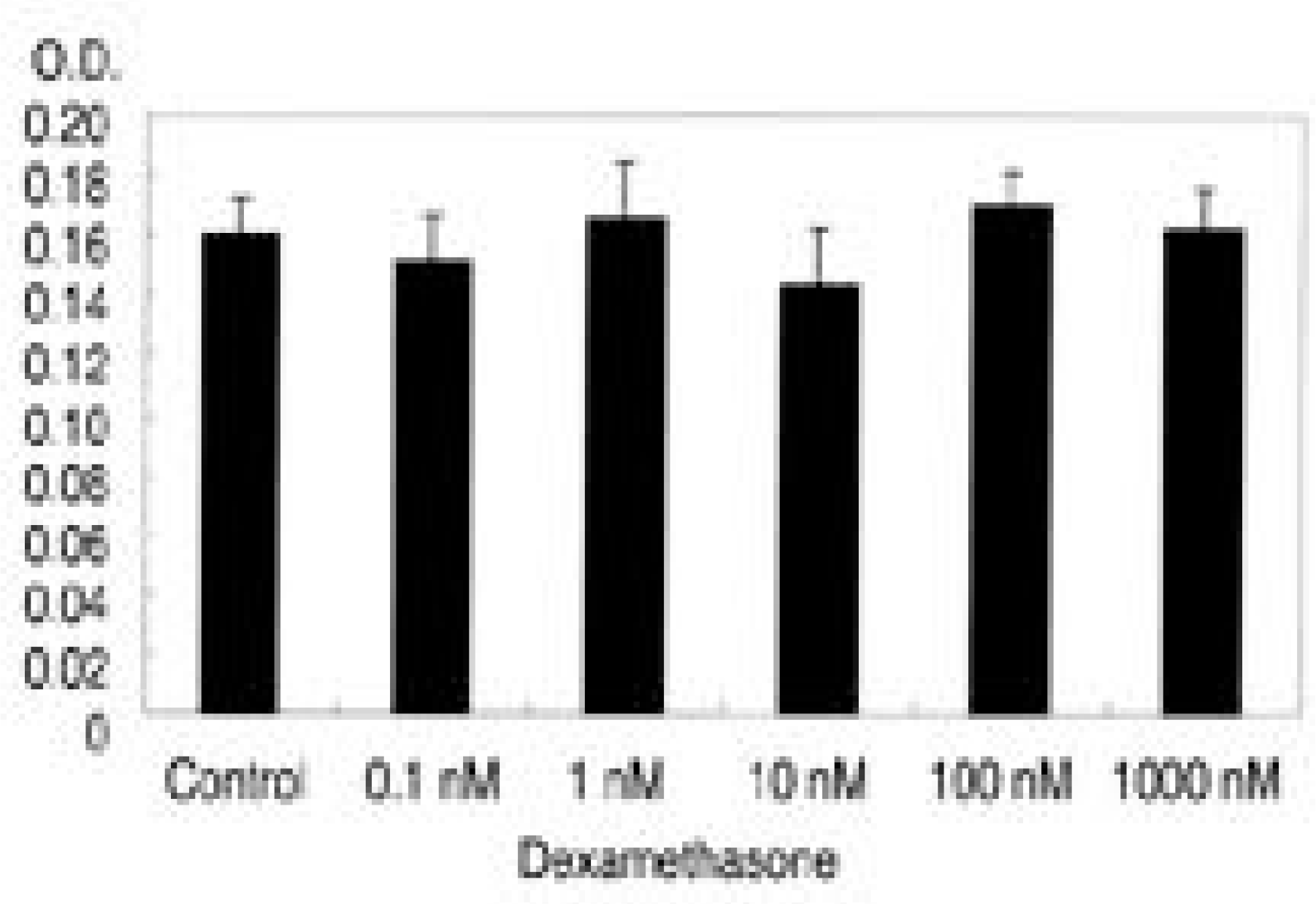 | Fig. 1.Survival of intervertebral disc cells with various concentration of dexamethasone(0.1, 1, 10, 100, 1000nM). There is no significant cytotoxicity of dexamethasone with dexamethasone compared to control. |
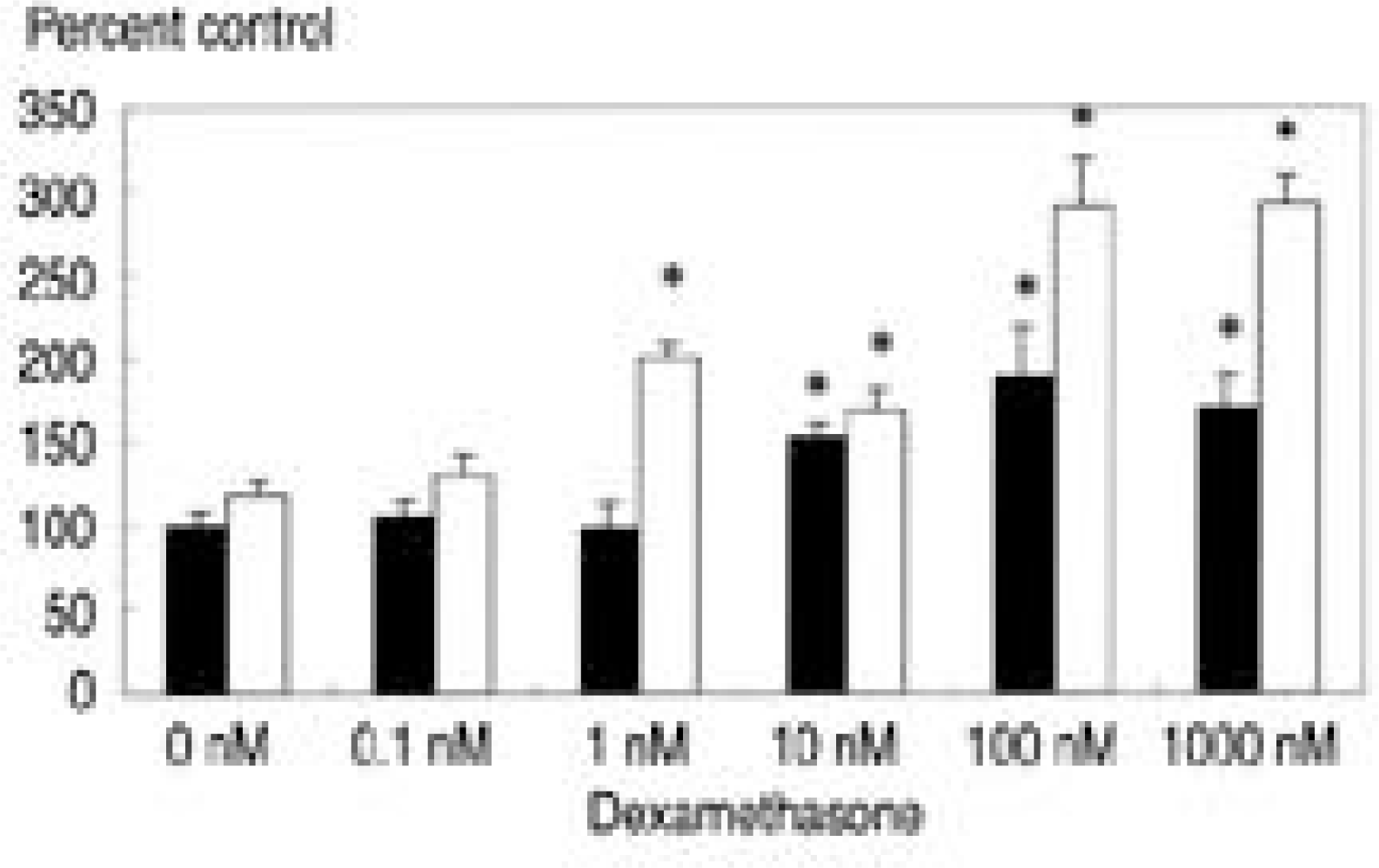 | Fig. 2.[3H]-thymidine incorporation with various dose of dexamethasone and TGF-β1(10 ng/ml). White bar denotes culture with TGF- β1. Dexamethasone with a concentration of 10 nM, 100 nM, and 1000 nM renders increased DNA synthesis compared to control(p<0.05). Culture with dexamethasone and TGF-β1(10 ng/ml) showed increased DNA synthesis from the 1nM of dexamethasone and also demonstrated synergistic effect in DNA synthesis. ∗ p<0.05 |
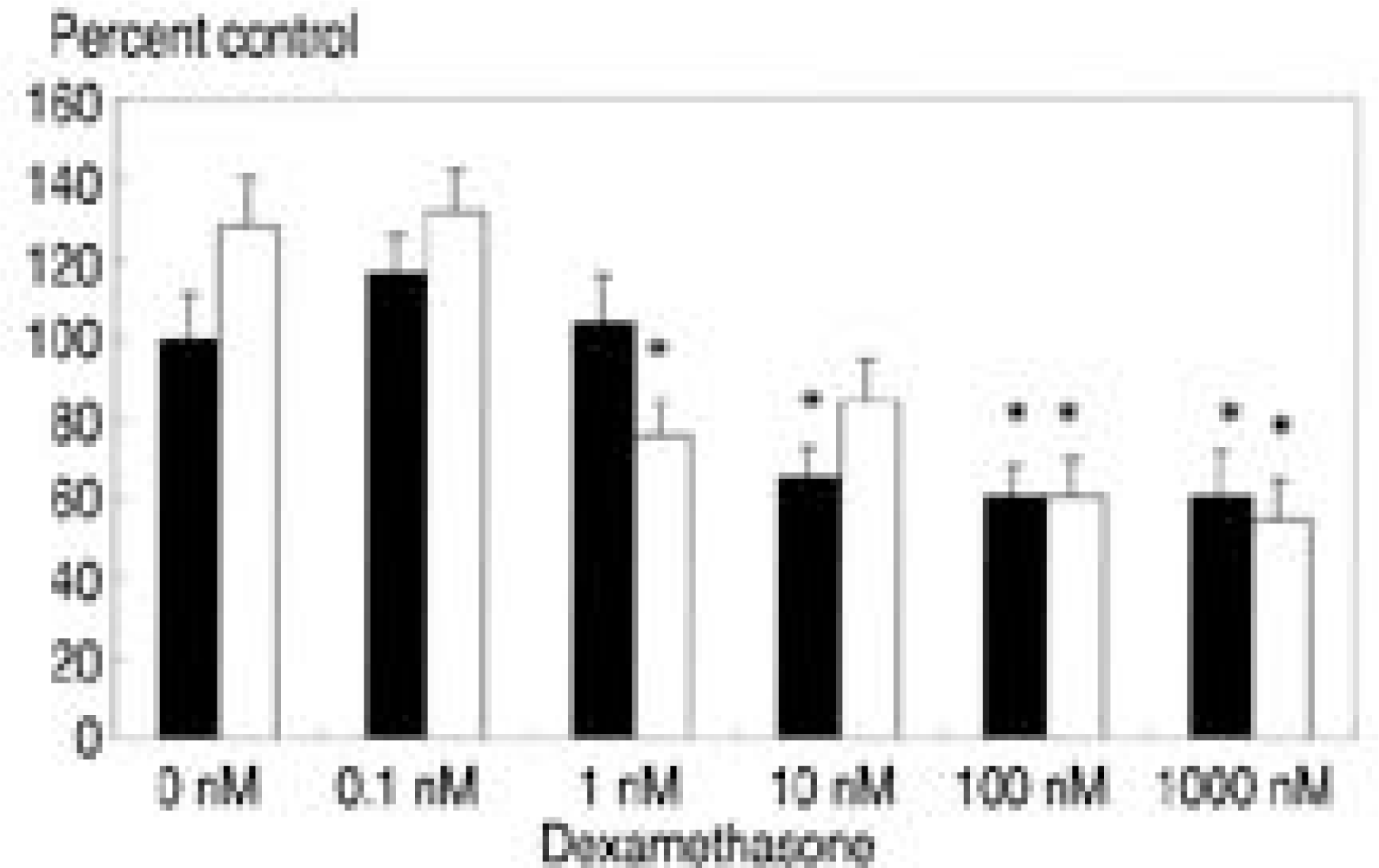 | Fig. 3.[35S]-sulfate incorporation with various dose of dexamethasone and TGF-β1(10 ng/ml). White bar denotes culture with TGF-β1. Dexamethasone with a concentration of 10 nM, 100 nM, and 1000 nM renders decreased proteoglycan synthesis compared to control (p<0.05). Culture with dexamethasone and TGF- β1(10 ng/ml) showed further decrease in proteoglycan synthesis at 1nM of dexamethasone. ∗ p<0.05 |
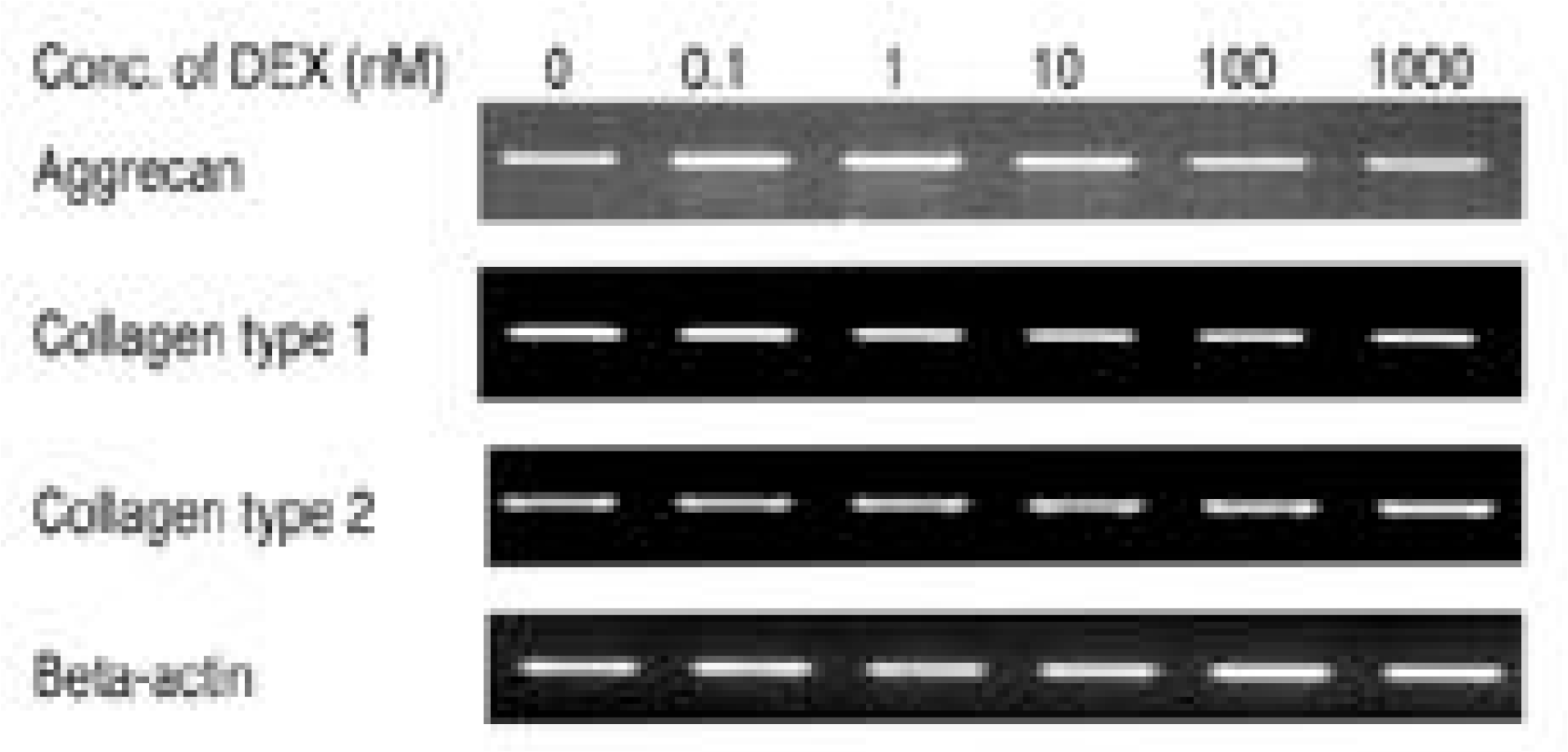 | Fig. 4.Expression of aggrecan, type I collagen, and type II collagen mRNA measured by reverse transcriptase poly-merase chain reaction. Cultures with various dose of dexamethasone showed no significant changes in the expression of aggrecan, type I collagen and type II collagen mRNA expression. Densitometric date was nor-malized by β-actin. |
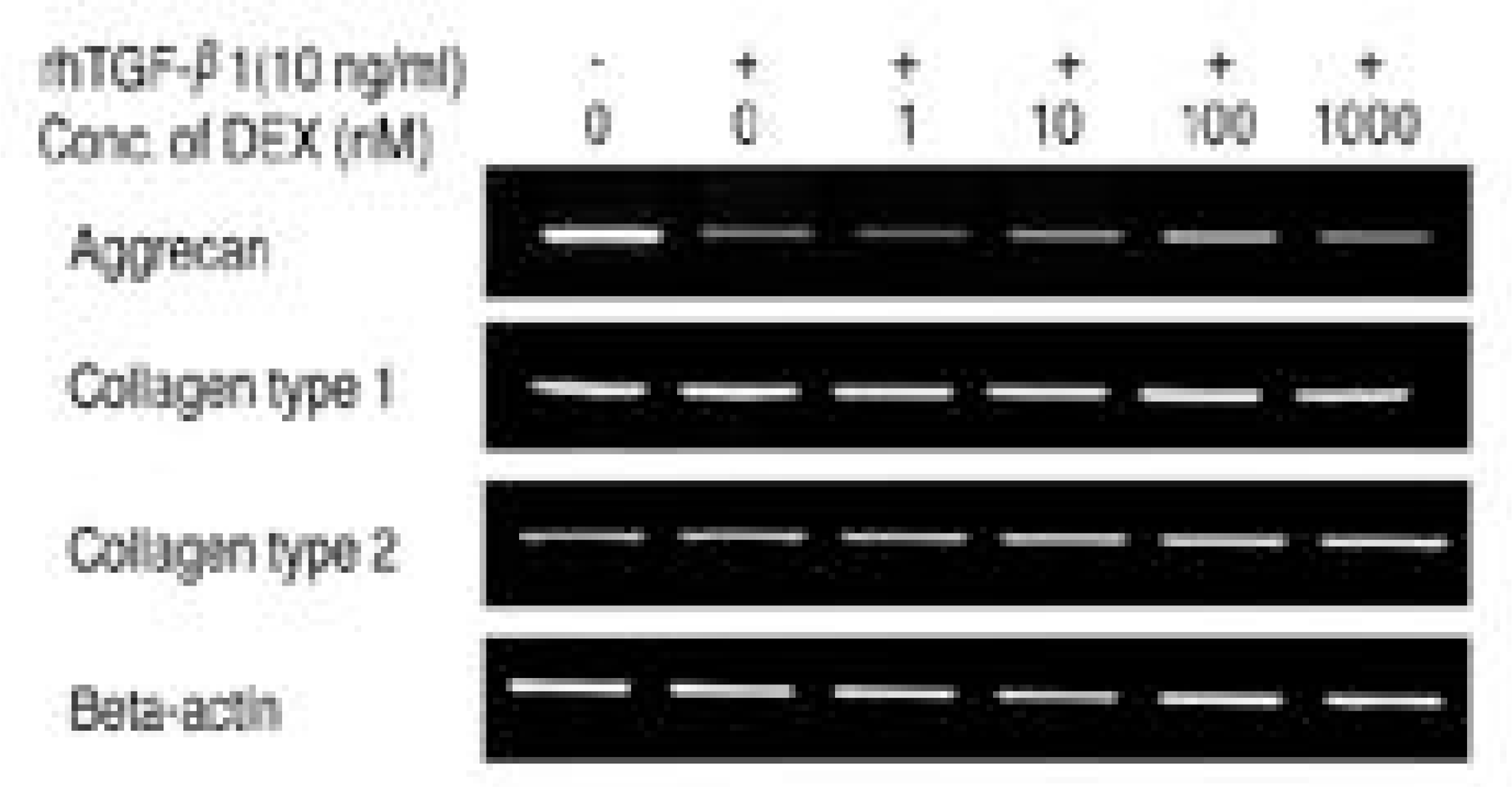 | Fig. 5.Expression of aggrecan, type I collagen, and type II collagen mRNA measured by reverse transcriptase poly-merase chain reaction. Cultures with various dose of dexamethasone and with TGF-β1(10 ng/ml) showed down-regulation of aggrecan mRNA expression while unchanged pattern in type I collagen and type II collagen mRNA expression. Densitometric date was normal-ized by β-actin. |
Table 1.
Sequences of the RT-PCR Primers Used




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download