Abstract
Study Design
This is a multicenter, randomized comparative outpatient study on a 8- week administration of Tramadol 37.5 mg/A cetaminophen and 325 mg (Tramadol/A PA P) combination tablets and Cyclo-Oxygenase- 2 inhibitor (Celecoxib).
Objectives
We wanted to evaluate the efficacy and safety of Tramadol/A PA P combination tablets and Celecoxib for the treatment of chronic low back pain.
Summary of the Literature Review
Tramadol/A PA P combination tablets have an analgesic efficacy for the treatment of chronic low back pain. The conditions for which COX - 2 inhibitors were be used included a variety of musculoskeletal conditions. However, further analyses are needed to determine the efficacy and safety of Tramadol/A PA P combination tablets and Celecoxib for the treatment of chronic low back pain.
Materials and Methods
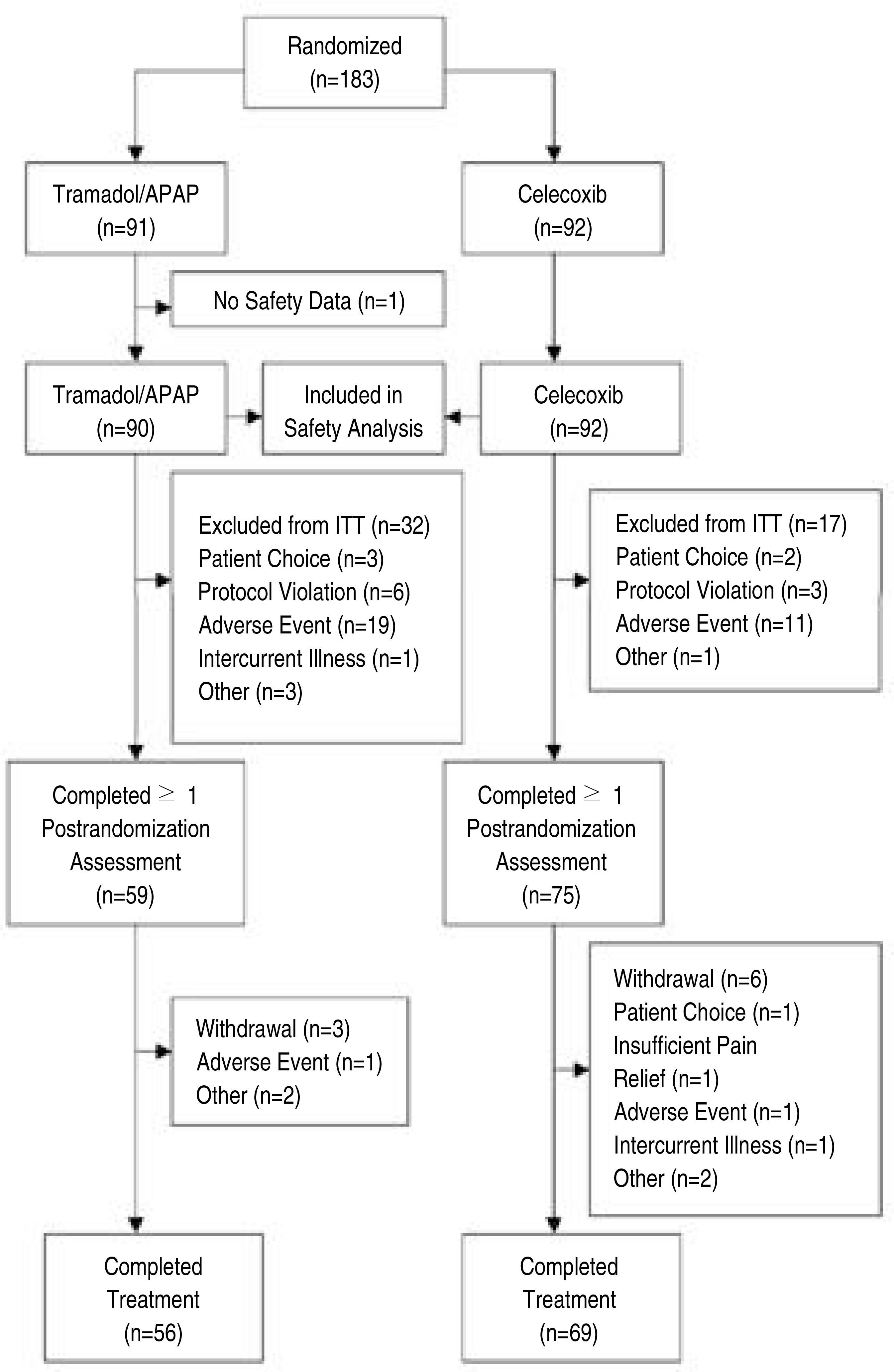
One hundred twenty- five patients with chronic low back pain (pain visual analogue scale [VA S] scores >40 mm on 100 mm scale) were randomized to take the Tramadol/A PA P combination tablets or Celecoxib for 8 weeks. The primary outcome measure was the pain VA S score, pain relief score and the Korean- version of Oswestry Disability Index (KODI).
Results
The study enrolled 125 patients (56 in the Tramadol/A PA P tablets group and 69 in the Celecoxib group). There were no significant differences between Tramadol/A PA P combination tablets and Celecoxib with regard to the pain V A S scores (VA S; 27.99± 21.22 vs 24.56± 16.58, respectively, p>0.05), the pain relief score and the mean decreased disability score on the KODI (0.42± .59 vs 0.46± .05, respectively). The adverse drug reactions showed a statistically significant difference (p<0.05).
Go to : 
REFERENCES
2). Patel AT, Ogle AA. Diagnosis and management of acute low back pain. Am Fam Physician. 2000; 61:1779–86.
3). Van Tulder MW, Scholten RJPM, Koes BW, Deyo RA. Nonsteroidal anti-inflammatory drugs for low back pain: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine. 2000; 25:2501–13.
4). Deyo RA. Drug therapy for back pain.: which drugs help which patients? Spine. 1996; 21:2840–50.
5). Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, Vaught JL. Opioid and nonopioid components inde -pendently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther. 1992; 260:275–85.
6). Schnitzer TJ, Gray WL, Paster RZ, Kamin M. Efficacy of tramadol in treatment of chronic low back pain. J Rheumatol. 2000; 27:772–8.
7). Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998; 50:1842–6.

8). Fleischmann RM, Caldwell JR, Roth SH, Tesser JRP, Olson W, Kamin M. Tramadol for the treatment of joint pain associated with osteoarthritis: a randomized, double-blind, placebo-controlled trial. Curr Ther Res. 2001; 62:113–28.

9). American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain. Glenview, IL: American Pain Society;2nd ed. 1999. p. 1–64.
10). Bjorkman R, Hallman KM, Hedner J, Hedner T, Hen-ning M. Acetaminophen blocks spinal hyperalgesia induced by NMDA and substance P. Pain. 1994; 57:259–64.
11). Muth-Selbach US, Tegeder I, Brune K, Geisslinger G. Acetaminophen inhibits spinal prostaglandin E2 release after peripheral noxious stimulation. Anesthesiology. 1999; 91:231–9.
12). Mullican WS, Lacy JR: TRAMAP-ANAG-006 Study Group. Tramadol/Acetaminophen Combination Tablets and Codeine/Acetaminophen Combination Capsules for the Management of Chronic Pain: A Comparative Trial. Clinical Therapeutics. 2001; 23:1429–1445.
13). Silverfield JC, Kamin M, Wu S-C, Rosenthal N. CAPSS-105 Study Group. Tr a madol/acetaminophen combination tablets for the treatment of osteoarthritis flare pain: a multicenter, outpatient, randomized, double-blind, placebo-controlled, parallel-group, add-on study. Clin Ther. 2002; 24:282–97.
14). Bennett RM, Kamin M, Karim R, Rosenthal N. Tramadol and acetaminophen combination tablets in the treatment of fibromyalgia pain: a double-blind, randomized, placebo-controlled study. Am J Med. 2003; 114:537–45.

15). Fricke JR Jr, Karim R, Jordan D, Rosenthal N. A dou -ble-blind, single-does comparison of the analgesic efficacy of tramadol/acetaminophen combination tablets, hydrocodone/acetaminophen combination tablets, and placebo after oral surgery. Clin Ther. 2002; 24:953–68.
16). Fortin L, Beaulieu A, Kamin M, Rosenthal N. Protocol TRP-CAN-1 Study Group. Analgesic Efficacy and Safety of Tramadol/Acetaminophen Combination Tabl et s(Ultra cetⓇ) in Treatment of Chronic Low Back Pain: A Multicenter, Outpatient, Randomized, Double Blind, Placebo Controlled Trial. The J Rheumatology. 2004; 31:2454–2463.
17). Ruoff GE, Rosenthal N, Jordan D, Karim R, Kamin M: Protocol CAPSS-112 Study Group. Tramadol/Acetaminophen Combination Tablets for the Treatment of Chronic Lower Back Pain: A Multicenter, Randomized, Double-Blind, Placebo-Controlled outpatient Study. 2003; 25:1123–1141.
18). Cox ER, Motheral B, Frisse M, Behm A, Mager D. Pre -scribing COX-2s for Patients New to Cyclo-oxygenase Inhibition Therapy. 2003; 9:735–742.
19). Bombardier C. An evidence-based evaluation of the gas -trointestinal safety of coxibs. Am J Cardiol. 2002; 89:3D–9D.
20). Katz N. The Impact of Pain Management on Quality of Life. J Pain Symptom Management. 2002; 24:38–47.

21). Savage R. Cyclo-Oxygenase-2 Inhibitors: When Should They Be Used in the Elderly? Drugs Aglng. 2005; 22:185–200.
22). Ruoff G, Lema M. Strategies in Pain Management: New and Potential Indications for COX-2 Specific Inhibitors. Journal of Pain and Symptom Management. 2003; 25:2S.
23). Jeon CH, Kim DJ, Kim DJ, Lee HM, Park HJ. Cross-cultural Adaptation of the Korean Version of the Oswestry Disability Index(ODI). J of Korean Spine Surg. 2005; 12:146–152.
24). Andersson GB. The epidemiology of spinal disorders. Frymoyer JW, editor. The Adult Spine: Principles and Practice. 2nd ed.Philadelphia;p. Lippincott–Raven. 1997. p. 93–141.
25). Andersson GB. Epidemiological features of chronic low back pain. Lancet. 1999; 354:581–5.
26). McPhillipsTangum CA, Cherkin DC, Rhodes LA, Markham C. Reasons for repeated medical visits among patient with chronic back pain. J Gen Intern Med. 1998; 13:289–295.
27). American Medical Directors Association. Chronic Pain Management in the Long-Term Care Setting. Clinical Practice Guideline. Baltimore, Md: American Medical Directors Association;1999.
28). Luo X, Pietrobon R, Curtis LH, Hey LA. Prescription of Nonsteroidal Anti-inflammatory Drugs and Muscle Relaxants for Back Pain in the United States. Spine. 2004; 29:E531–E537.

29). Savage R. Cyclo-Oxygenase-2 Inhibitors: When Should They Be Used in the Elderly? Drugs Aging. 2005; 22:185–200.
30). Ruoff G, Lema M. Strategies in Pain Management: New and Potential Indications for COX-2 Specific Inhibitors. Journal of Pain and Symptom Management. 2003; 25:2S.
31). Curtis SP, Ng J, Yu Q, Shingo S, Bergman G, McCormick CL, Reicin AS. Renal Effects of Etoricoxib and Comparator Nonsteroidal Anti-Inflammatory Drugs in Controlled Clinical Trials. Clin Ther. 2004; 1:70–83.

32). Brater DC, Harris C, Redfern JS, Gertz BJ. Renal effects of COX-2 selective inhibitors. Am J Nephrol. 2001; 21:1–15.
33). Savage RL. A dangerous trio. Prescriber Update. 2002; 23:20.
Go to : 
Table 1.
Demographic Data
Table 2.
Evaluation of Pain Intensity
Table 3.
Evaluation of Changes of KODI
| GROUP | N | Mean (%) | Std Dev (%) |
|---|---|---|---|
| Tramadol/APAP | 56 | 0.42 (21.55%) | 0.59 (32.64%) |
| Celecoxib | 69 | 0.46 (27.53%) | 0.50 (27.03%) |
Table 4.
Overall Evaluation of the KODI




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download