Abstract
Study Design
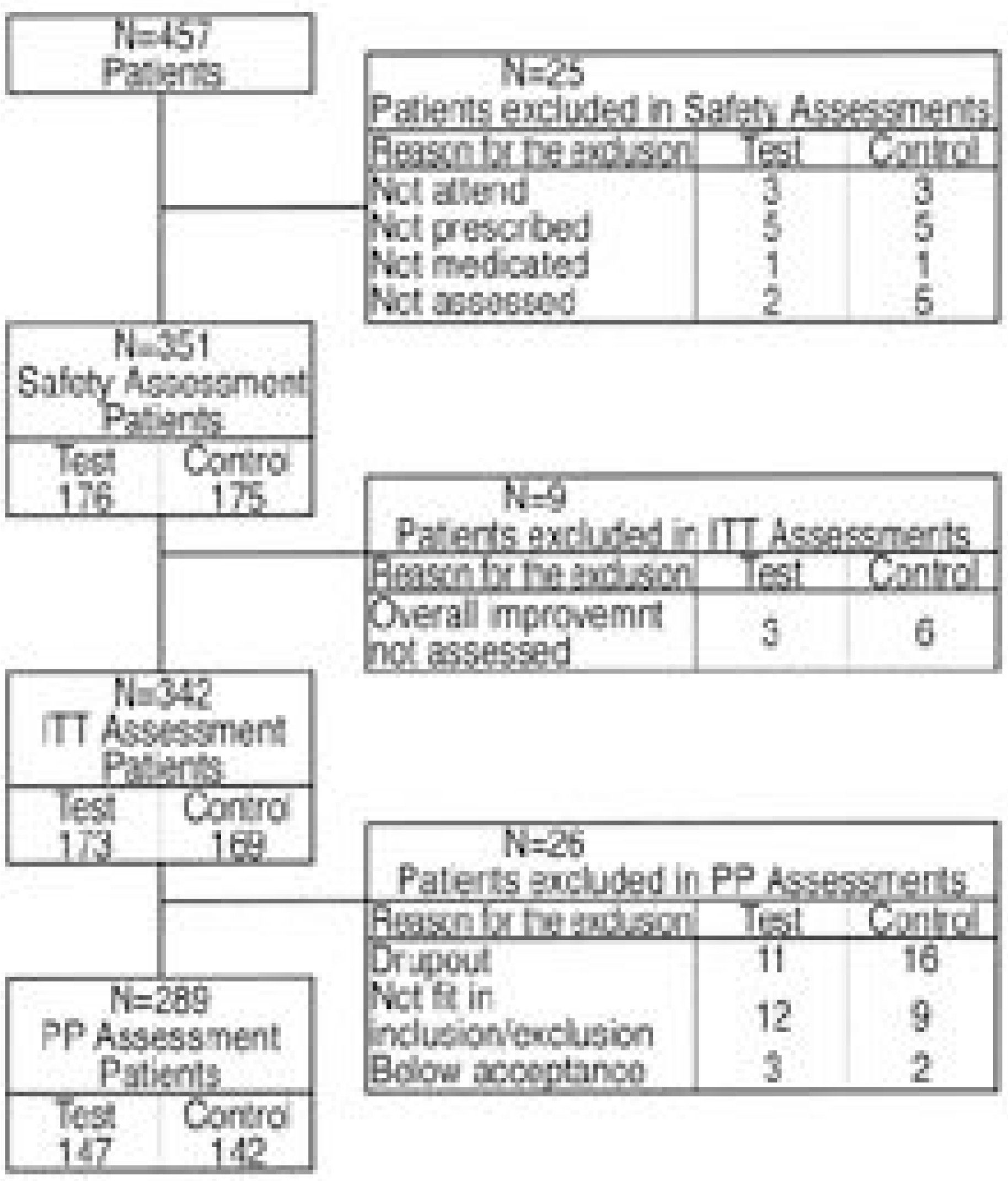
A Multicenter doubleblind randomized clinical study comparing Neurotropin and A ceclofenac.
Objective
To evaluate the analgesic effect, efficacy and safety of Neurotropin in patients with low back pain.
Summary of Literature Review
Non steroidal anti inflammatory analgesics are used as the main medical treatment in patients with low back pain. However, complications, such as gastrointestinal or cardiovascular problems, have been well documented. Neurotropin acts to recover the analgesic state arising from a decrease in pain threshold and has a completely different mechanism to that of existing anti-inflammatory and narcotic analgesics, with its action of restoring the immune system having been confirmed.
Materials & Method
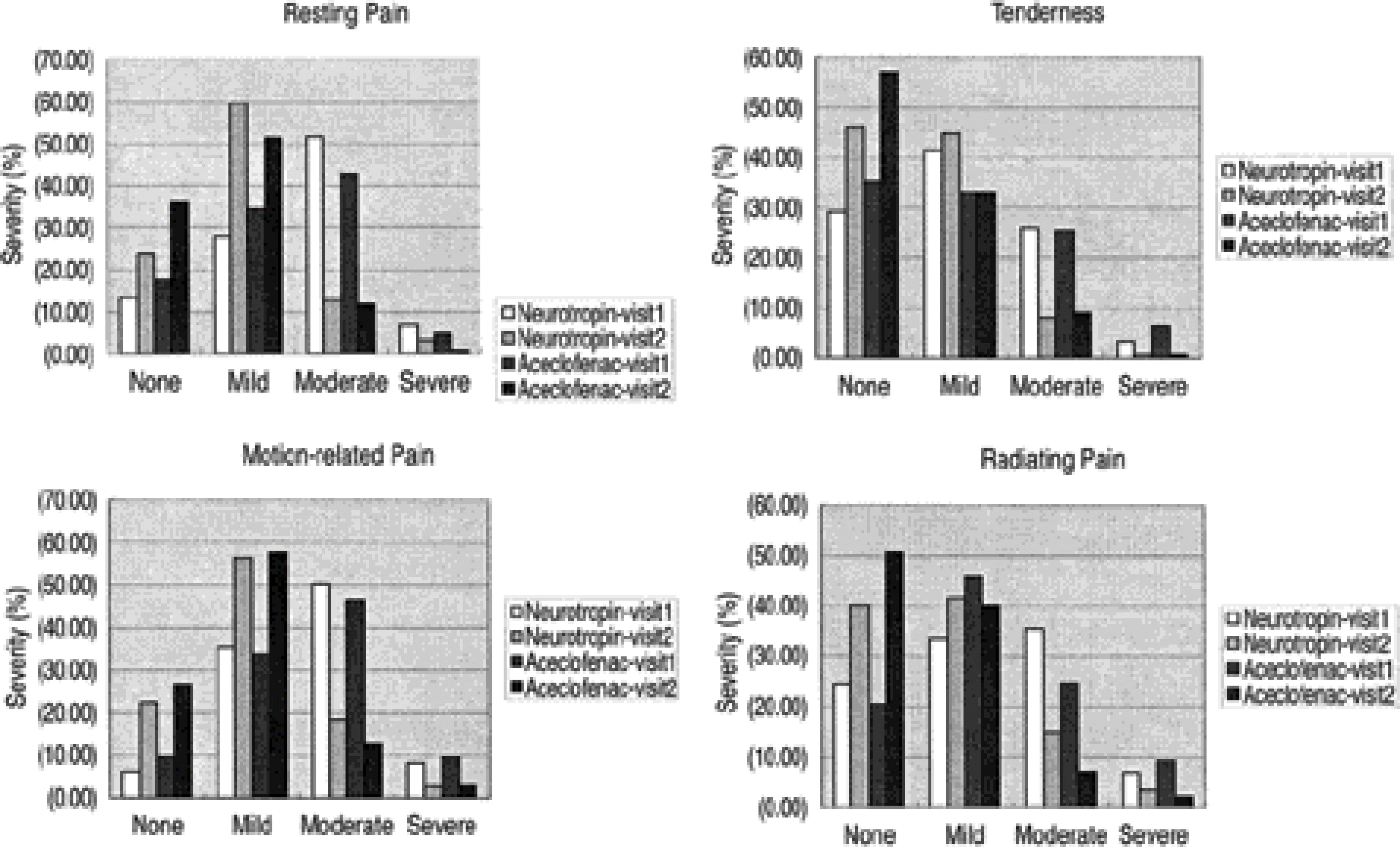
376 patients with back pain were randomly divided into two groups; one group was administered Neurotropin and the other A ceclofenac. The overall improvement after 4 weeks was used as the first efficacy variable, and with the second efficacy variable the improvements in spontaneous pain, tenderness, motion pain, radiating pain, severity, pain intensity, and the overall severity and Oswestry Disability Indices were used as the evaluation criteria. To evaluate safety, the abnormal clinical response and alternations on physical examination and the clinical laboratory values were used.
Results
A total of 358 patients received the experimental and comparison drugs, of which 351 were evaluated for safety. The overall improvement after 4 weeks, severity of symptoms, overall severity, and the pain intensity and Oswestry Disability Indices were decreased in both groups, but the differences between the two groups were not statistically significant. The overall decrease in the severity was greater in the Aceclofenac group, but both groups had statistically meaningful decreases after the administration of the drugs. i.e. Adverse drug reactions were less in the Neurotropin group, but these showed no significant statistical difference.
Go to : 
REFERENCES
3). Watson DJ, Harper SE, Zhao PL, et al. Gastrointestinal tolerability of the selective cyclooxygenase-2 (COX-2) inhibitor rofecoxib compared with non-selective COX-a and COX-2 inhibitors in osteoarthritis. Arch Intern Med. 2000; 160:2998–3003.
4). Hickey RF. Chronic low back pain: a comparison of diflunisal with paracetamol. New Zeal Med J. 1982; 95:312–314.
6). Katz N, Ju WD, Krupa DA, et al. Efficacy and safety of rofecoxib in patients with chronic low back pain: results from two 4-week, randomized, placebo-controlled, paral -lel-group, double-blind trials. Spine. 2003; 28:851–858.
7). Chrubasik S, Model A, Black A, Pollak S. A randomized double-blind pilot study comparing Doloteffin and Vioxx in the treatment of low back pain. Rheumatology. 2003; 42:141–148.
8). Miura T, Okazaki R, Yoshida H, Namba H, Okai H, Kawamura M. Mechanisms of analgesic action of Neur otropinⓇon chronic pain in adjuvant-induced arthritic rat: roles of descending noradrenergic and serotonergic systems. J Pharmacol Sci. 2005; 97:429–436.
9). Sobue I, Tashiro K, Hanakago R, et al. Clinical evaluation of NeurotropinⓇinjection on dysesthesia of SMON (subacute myelo-optico-neuropathy)-a multi-institutional double-blind comparative study-. J Clin Ther Med. 1992; 8:833–851.
10). Ning G, Zou DJ, Liu W, et al. Multicenter, randomized, positive-controlled clinical study for the effects of NeurotropinⓇon diabetic neuropathy. Zhonghua Yi Xue Za Zhi. 2004; 84:1785–1787.
Go to : 
Table 1.
Demographic/Baseline Data
Table 2.
Efficacy Evaluation (CGI Response Rate)-PP
Table 3.
Efficacy Evaluation (CGI Response Rate)-PP
Table 4.
Efficacy Evaluation ((Pain Intensity evaluated by VAS)-PP
Table 5.
Efficacy Evaluation (Overall Severity & Oswestry Scale)-PP
Table 6.
Safety Evaluation




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download