Abstract
Objectives
To compare the biodegradation and osteoconduction properties of CaO- SiO2- P2 O5- B2 O3 glass- ceramics and CeraboneⓇ- A W.
Summary of Literature Review
Bioglass ceramics can be used as bone graft substitutes. However, no study has been undertak-en to investigate the possibility of CaO- SiO2- P2 O5- B2 O3 glass- ceramics as a bone graft substitute.
Materials and Methods
Porous CSPB2 implants (44.07% CaO, 40.28% SiO2, 8.1% P2 O5 and 5.0% B 2 O3), porous CSPB3 implants (43.76% CaO, 43.41% SiO2, 4.05% P2O5 and 7.5% B2O3) and porous CeraboneⓇ- AW were prepared by the polymer sponge method. Single- level posterolateral spinal fusions were performed on sixty New Zealand white male rabbits. The animals were divided into four groups (9 of autograft, 17 per 3 kind of porous implant group) according to the implant material used: autograft, CSPB2, CSPB3 and CeraboneⓇ- A W. Radiographs were performed every two weeks. All animals were sacrificed 12 weeks after surgery. Manual palpation and uniaxial tensile strength were determined. The proportion of the area occupied by the ceramics in the final compared to the initial radiographs was calculated. Decalcified and undecalcified histological sections were evaluated by light microscopy.
Results
Fifty one rabbits were evaluated. The union rates were 100 (9 out of 9), 80 (8 out of 8), 81.1(9 out of 11) and 90.9% (10 out of 11) in the autograft, CeraboneⓇ- AW, CSPB2 and CSPB3 groups, respectively. The proportion of the area occupied by CeraboneⓇ- A W (9 0.8%± 14.0) was significantly higher than for CSPB2 (73.1%± 11.5) and CSPB3 (73.5%± 10.0)(p=0.0011). The mean values of the tensile strengths of CeraboneⓇ- AW (214. ± 57.3N), CSPB2 (214.± 57.3 N) and CSPB3 (217± 70.1N) were not significantly different (p>0.05).
Conclusion
CSPB2 and CSPB3 had similar tensile strengths and fusion rates of the fusion masses as those of CeraboneⓇ- A W; however, they degraded more rapidly than CeraboneⓇ- A W. These findings suggest that CSPB2 and CSPB3 grafts can be used as a more ideal new bone graft substitutes than CeraboneⓇ- A W.
Go to : 
REFERENCES
1). Klein CP, Driessen AA, de Groot K, Van Den Hooff A. Biodegradation behavior of various calcium phosphate materials in bone tissue. J Biomed Mat Res. 1983; 17:769–784.

2). Hench LL, Wilson JW. Surface-active Biomaterial. Sci -ence. 1984; 226:630.
3). Hench LL, Paschall HA. Histo-chemical responses at a biomaterials interface. J Biomed Mater Res. 1974; 5:49–64.

4). Hench LL: Bioceramics. From concept to clinic. J Am Ceram Soc. 1991; 81:1497–1510.
5). Kokubo T, Ito S, Sakka S, Yamamuro T. Formation of a high-strength bioactive glass-ceramic in the system MgO-CaO-SiO2-P2O5. J Mater Sci. 1986; 21:536–540.
6). Lee JH, Ha JH, Lee DH, et al. Evaluation of biodegradation and osteosynthesis in CaO-SiO2-B2 O3 glass-ceram -ics by posterolateral fusion of rabbit lumbar vertebrae. J Kor Orthop Assoc. 2003; 38:347–353.
7). Ryu HS, Seo JH, Kim H, et al. Preparation of CaO-SiO2- B2 O3 glass-ceramics and evaluation of bioactivity using in-vitro test. J Kor Ceramic Soc. 2002; 39:490–497.
8). Boden SD, Schimandle JH, Hutton WC. An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine. 1995; 20:412–420.
9). Glazer PA, Heilmann MR, Lotz JC, Bradford DS. Use of electromagnetic fields in a spinal fusion: A rabbit model. Spine. 1997; 22:2351–2365.
10). Holmes RE, Bucholz RW, Mooney V. Porous hydroxyapatite as a bone graft substitute in diaphyseal defects: a histometric study. J Orthop Res. 1987; 5:114–121.

11). Uchida A, Nada SM, McCartney ER, Ching W. The use of ceramics for bone replacement. A comparative study of three different porous ceramics. J Bone Joint Surg. 1984; 66-B:269–275.

12). Eggli PS, Muller W, Schenk RK. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. Clin Orthop. 1988; 232:127–138.

13). Blokhuis TJ, Termaat MF, den Boer FC, Patka P, Bakker FC, Haarman HJ. Properties of calcium phosphate ceramics in relation to their in vivo behavior. J Trauma. 2000; 48(1):179–186.

14). Lee JH, Lee DH, Ryu HS, et al. Porous beta-calcium pyrophosphate as a bone graft substitute in a canine bone defect model. Key Eng Mat. 2003; 240-242:399–402.

15). Boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine. 2002; 27:S26–S31.

Go to : 
Figures and Tables%
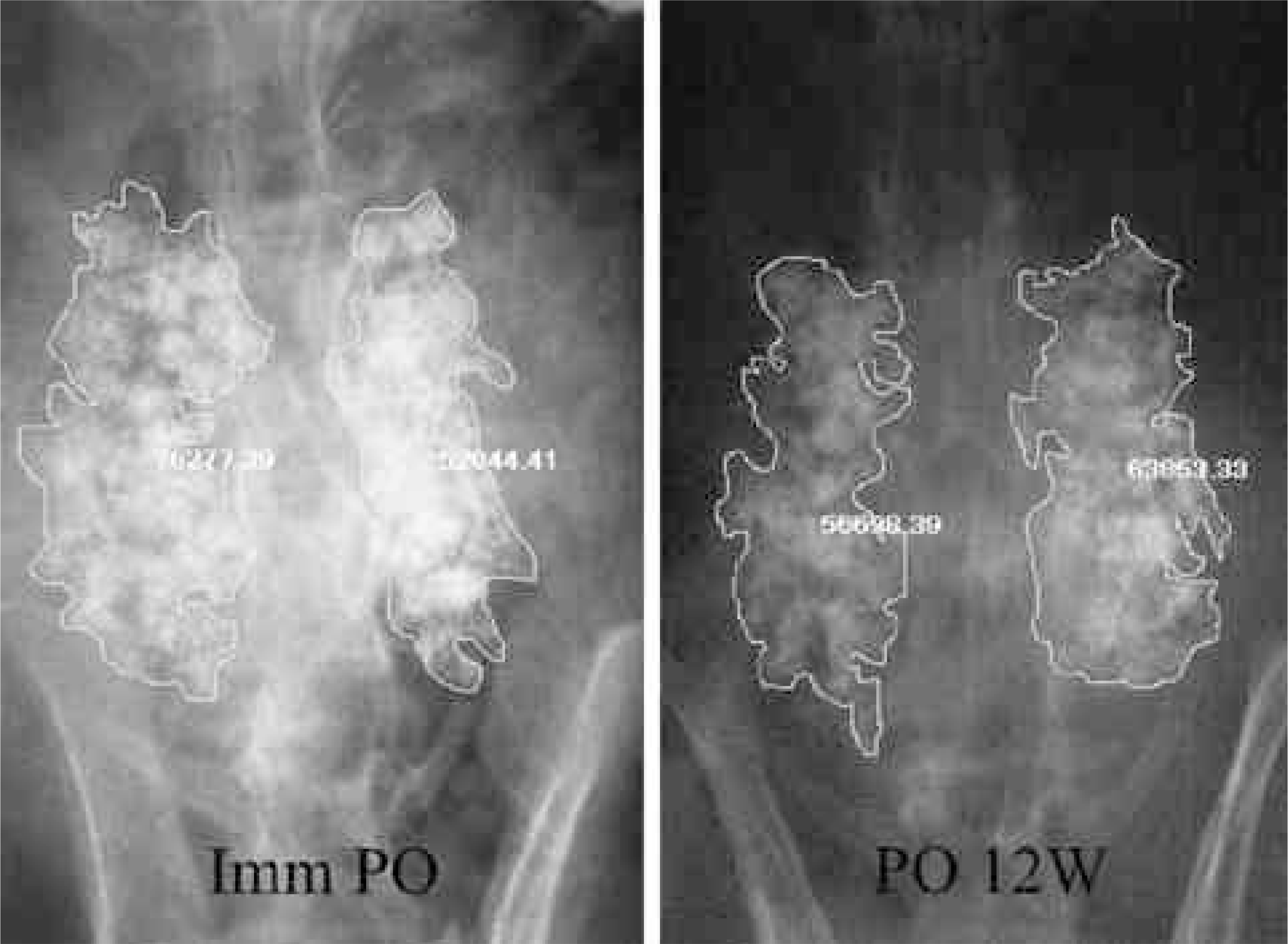 | Fig. 1.The calculation process using Image-ProⓇ Plus (Media Cybernetics, USA). Left: Immediate postoperative radiograph, Right: postoperative 12 weeks radiograph. |
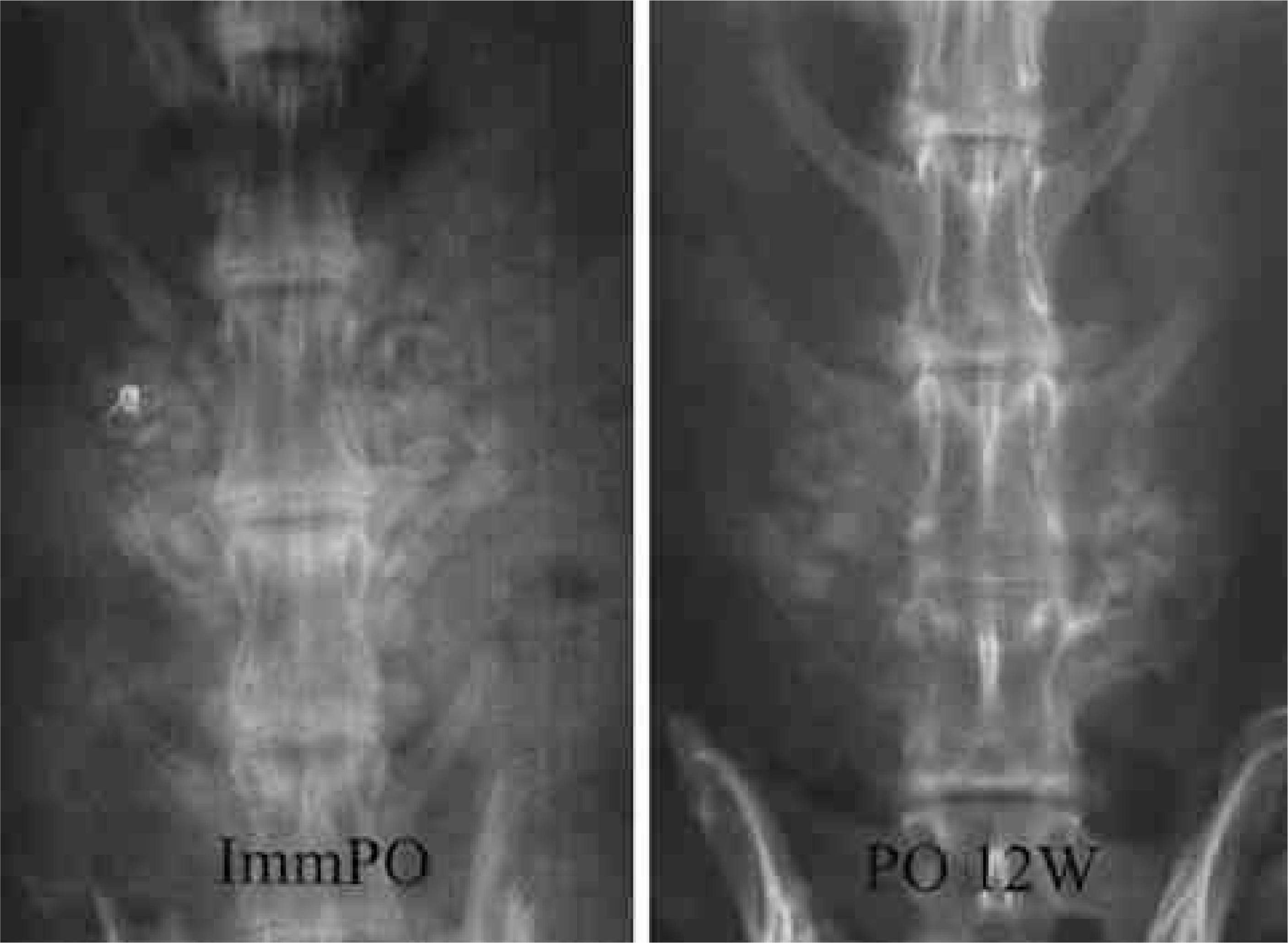 | Fig. 2.Posteroanterior radiographs of a rabbit in the autograft group. At 12 weeks, homogeneous fusion masses were formed. |
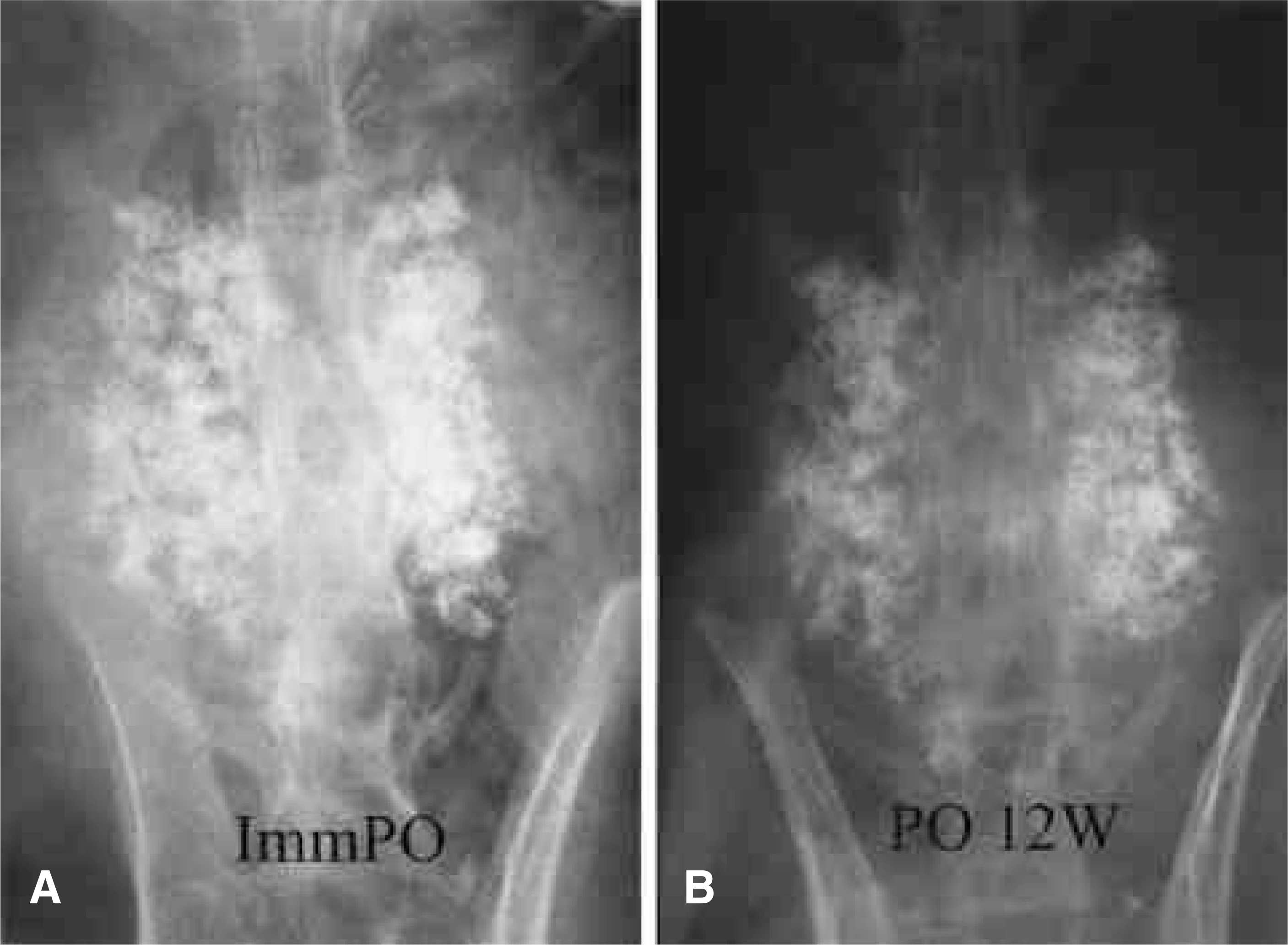 | Fig. 3.Posteroanterior radiographs of a rabbit in the CeraboneⓇ A-W group. At 12 weeks, fusion masses seemed to be formed, but the porous structure of the graft was maintained. The proportion of the area occupied by CeraboneⓇ A-W in final radiograph over the area occupied by ceramics in the initial radiograph was almost the same. (A) Immediate postoperative, (B) postoperative 12 weeks. |
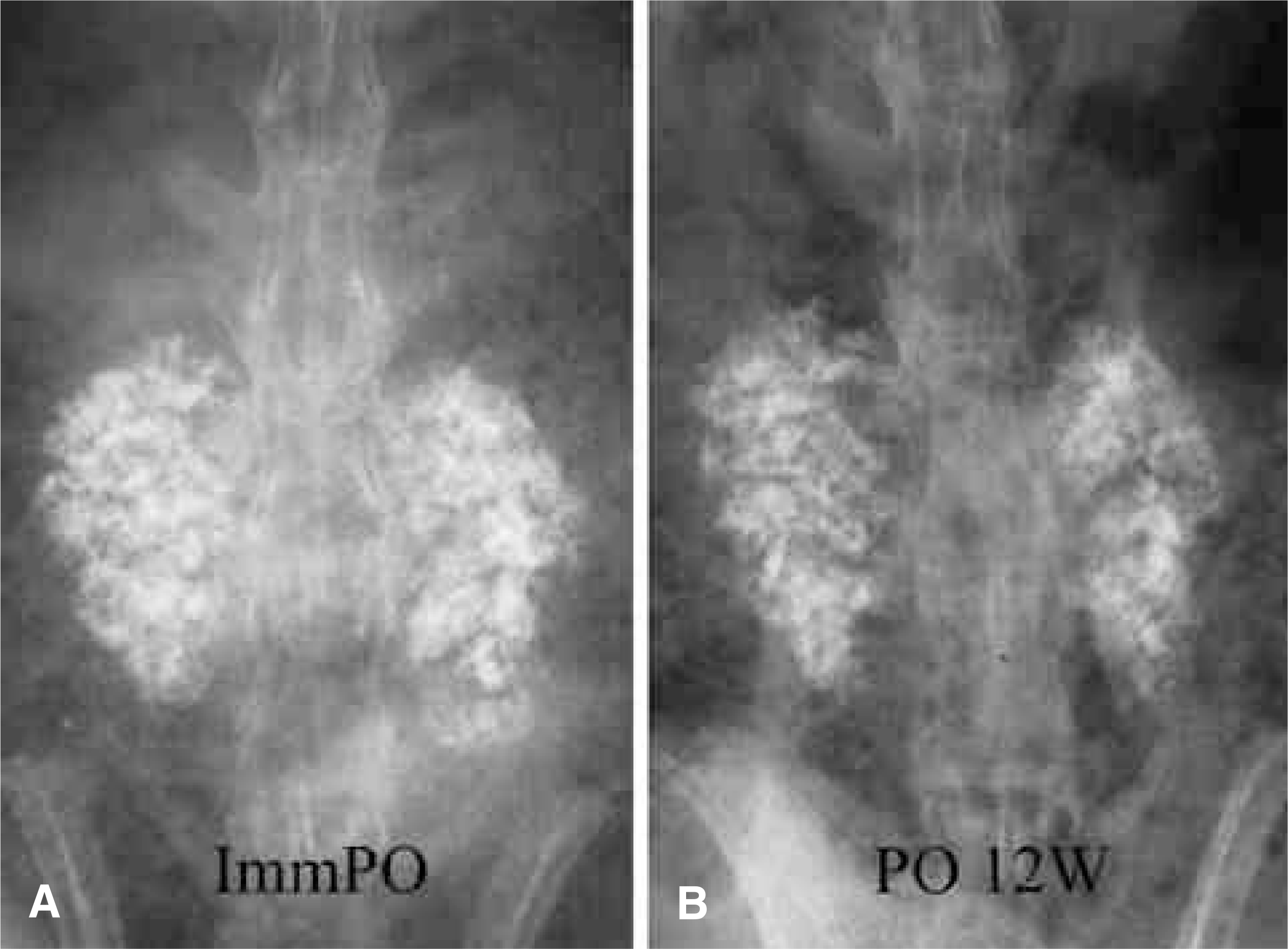 | Fig. 4.Posteroanterior radiographs of a rabbit in the CSPB2 group. (A) Immediate postoperative, (B) postoperative 12 weeks. |
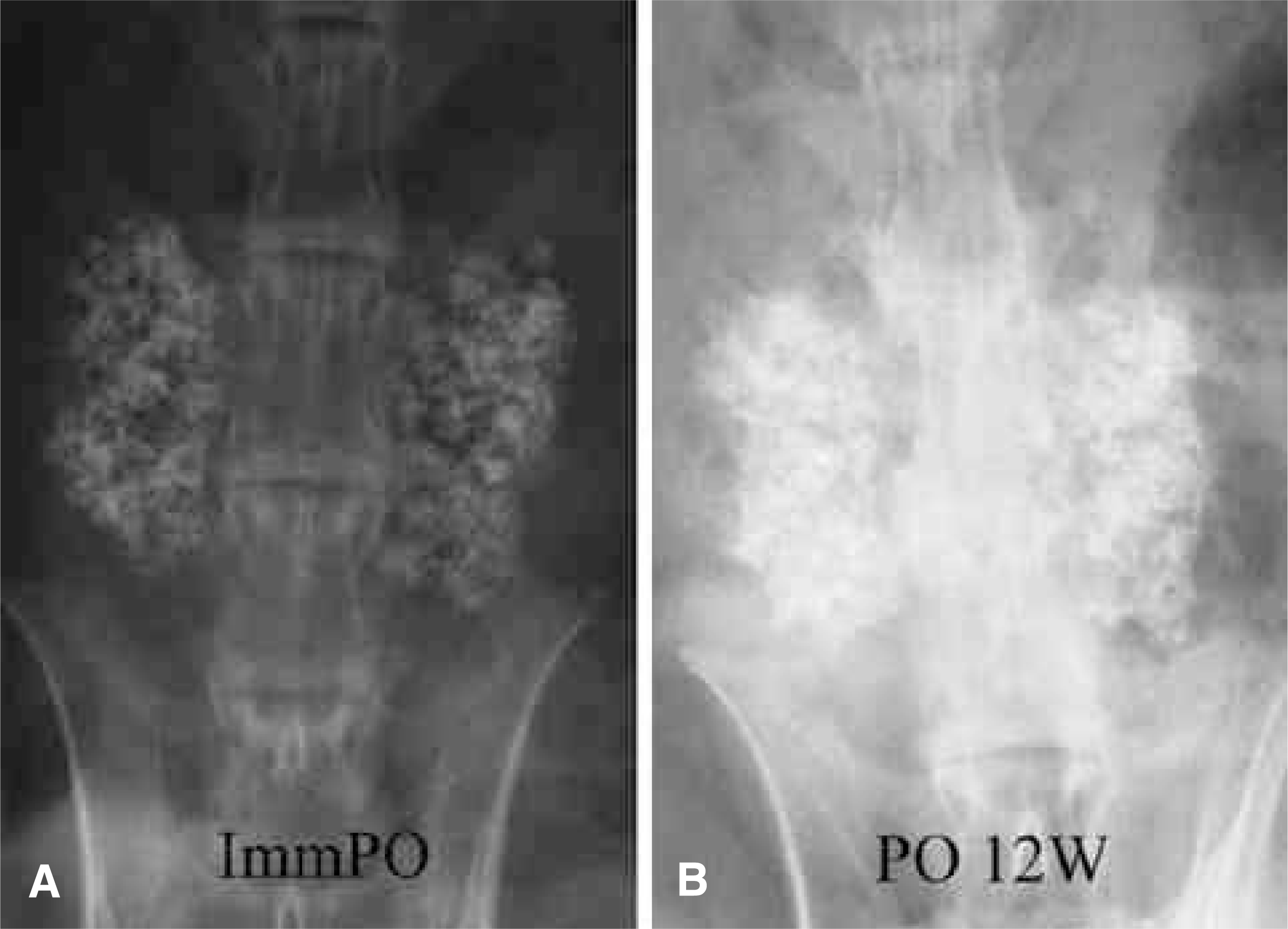 | Fig. 5.Posteroanterior radiographs of a rabbit in the CSPB3 group. (A) Immediate postoperative, (B) postoperative 12 weeks. |
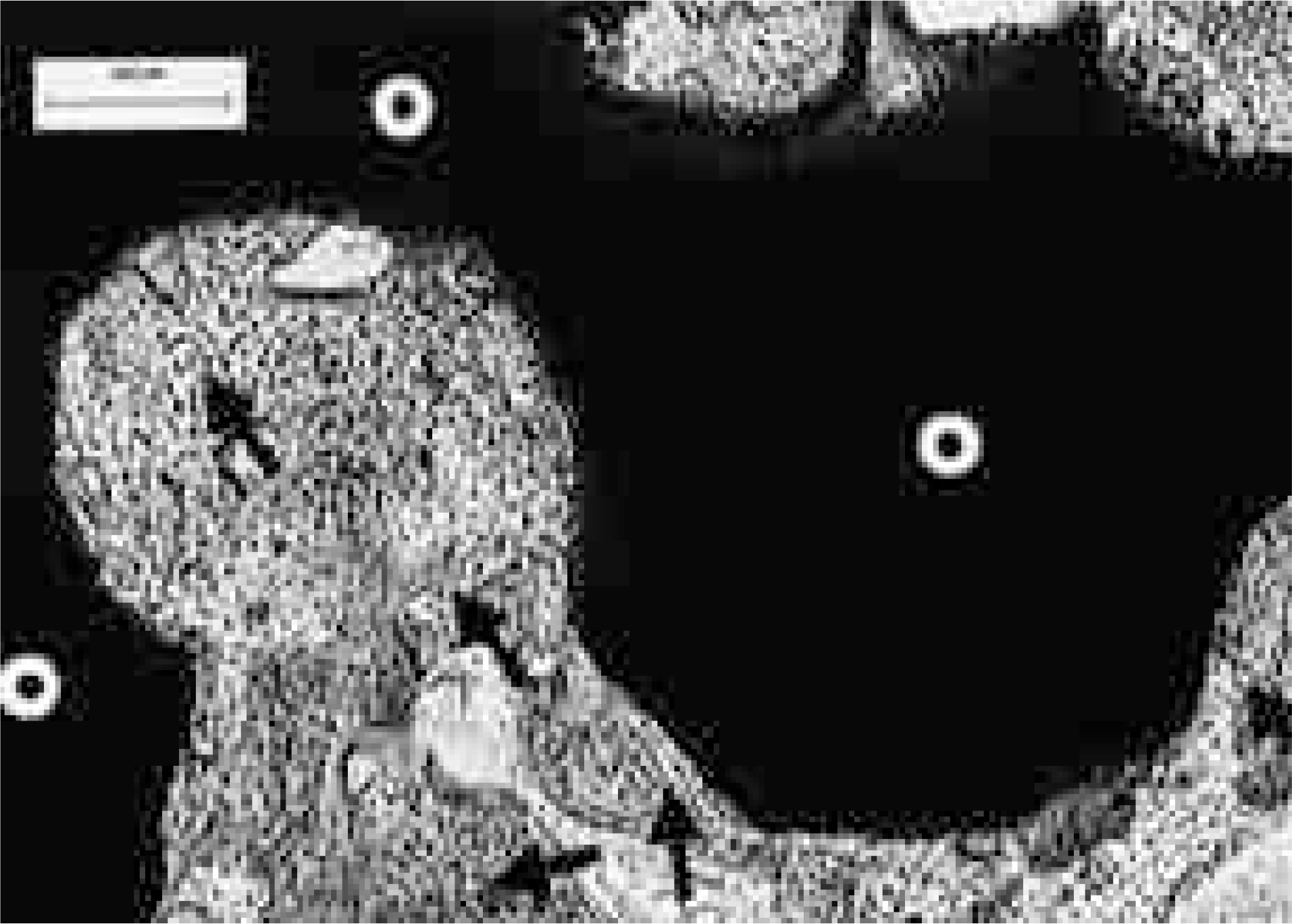 | Fig. 6.Histologic results of CeraboneⓇ A-W 12 weeks after surgery. The bone is directly attached to pores, the contours of which are nearly intact (Undecalcified, H&E staining, × 40). White circles indicate the CeraboneⓇ A-W implant, black arrows indicate newly formed bone. |
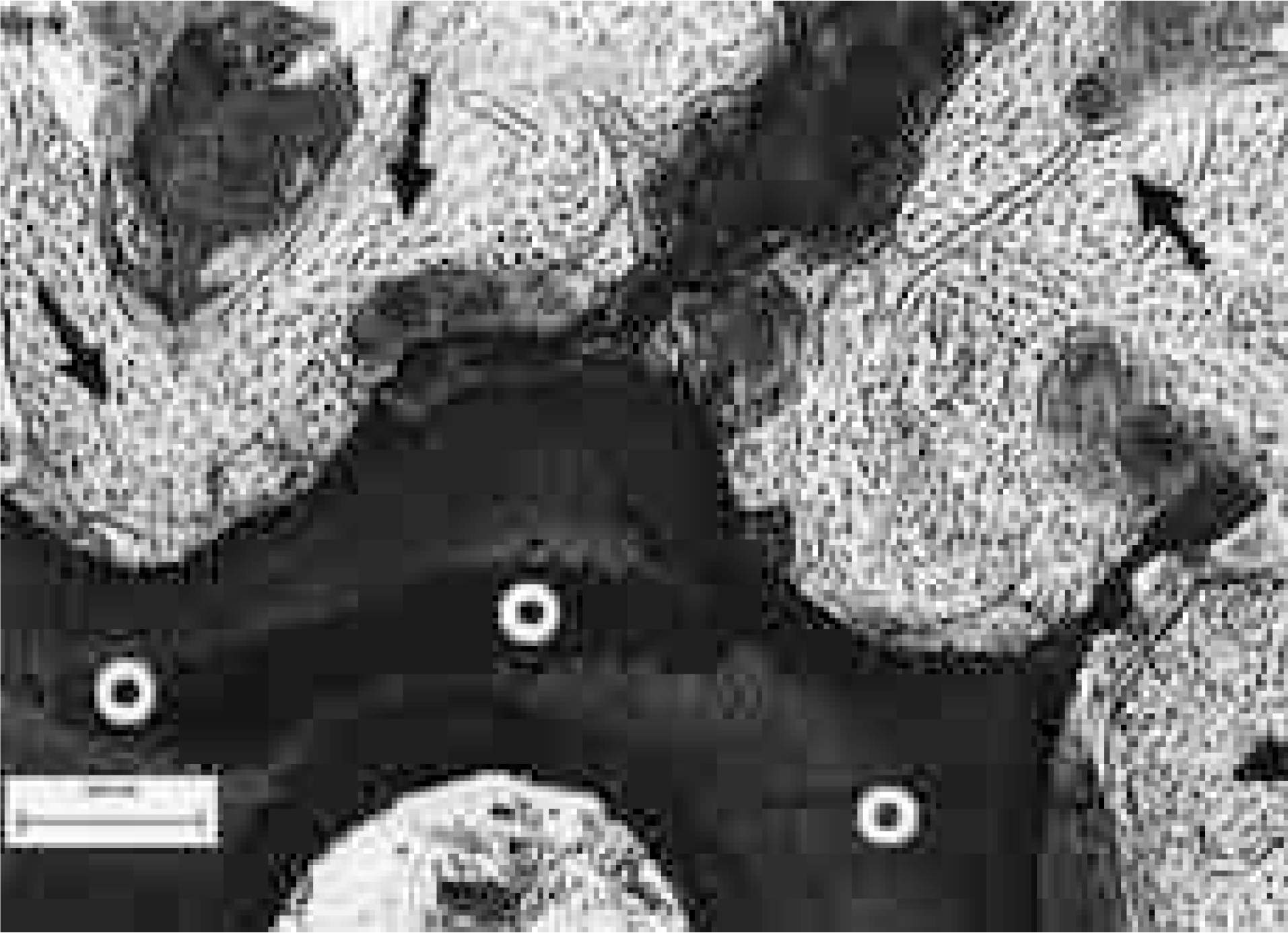 | Fig. 7.Histologic results of CSPB2 12 weeks after surgery. The bone is directly attached to pores, the contours of which are nearly intact (Undecalcified, H&E staining,× 40). White circles indicates the CSPB2 implant, black arrows indicate newly formed bone. |
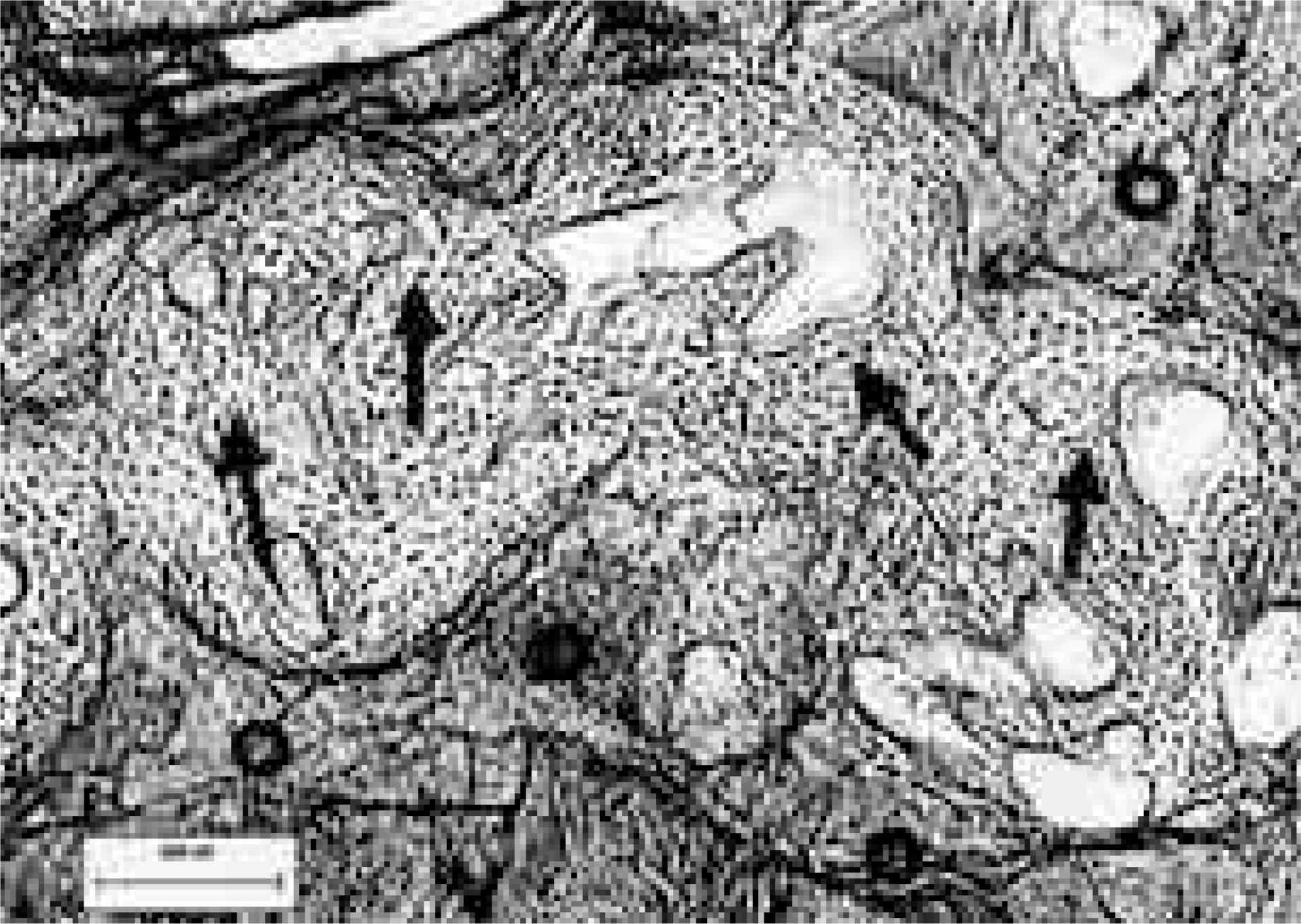 | Fig. 8.Histologic results of CSPB3 12 weeks after surgery. The bone is directly attached to pores, the contours of which are nearly intact (Undecalcified, H&E staining,× 40). White circles indicates the CSPB3 implant, black arrows indicate newly formed bone. |
Table 1.
Summary of fusion rate, radiomorphometric of lumbar intertransverse process fusions, and ultimate tensile strength.
| Fusion rate (%) | Proportion of area occupied by ceramics in final X-ray (%) | mean tensile strength (N) | |
|---|---|---|---|
| Cerabone-AW | 80 | 90.8± 14.0 a | 214.± 57.3 |
| CSPB2 | 81.8 | 73.1± 11.5 aa | 217± 66.1 |
| CSPB3 | 90.9 | 73.5± 10.0 aa | 217± 70.1 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download