Abstract
Study Design
In- vitro experiments using human mesenchymal stem cells (MSCs), intervertebral disc (IVD) cells and type 5 adenovirus/transforming growth factor- β1 construct (A d/TGF- β1).
Summary of Literature Review
MSCs are known to be multipotent in tissue regeneration. In degeneration of IVD, cellular replacement with genetic modification other than that of IVD cells may prove an enhanced mechanism for the regeneration of IVD cells.
Materials and Methods
MSCs and IVD cells were cultured and an adenovirus construct containing TGF- β1 cDNA (A d/TGF- β 1) was also produced. In the first step, the MSCs were transduced with A d/TGF- β1, then mixed with IVD cells in various proportions and three dimensionally cultured. [methyl-3 H]Thymidine and [35 S]Sulfur incorporation for DNA and proteoglycan synthesis, respectively, were measured. RT-PCR was performed to assess the aggrecan and collagen types I and II mRNA expressions
Results
Mixed cultures of MSC and IVD cells showed relatively similar amounts of newly synthesized proteoglycan compared with cultures of IVD cells only. In mixed cultures transduced with A d/TGF- β1, there were significant decreases in newly synthesized proteoglycan with increasing the proportions of MSCs, which was also found with the aggrecan and collagen type II mRNA expressions. However, the collagen type I mRNA expression increased with increased proportions of MSCs transduced with A d/TGF- β1.
Go to : 
REFERENCES
1). Borenstein D. Epidemiology, etiology, diagnostic evaluation, and treatment of low back pain. Curr Opin Rheuma -tol. 1992; 4:226–32.

2). Hardingham TE, Adams P. A method for the determination of hyaluronate in the presence of other glycosamino -glycans and its application to human intervertebral discs. Biochem J. 1976; 159:143–147.
3). Pearce RH, Grimmer BJ, Adams ME. Degeneration and the chemical composition of the human lumbar intervertebral disc. J Orthop Res. 1987; 5:198–205.

4). Lipson SJ, Muir H. Proteoglycans in experimental intervertebral disc degeneration. Spine. 1981; 6:194–210.

5). Melrose J, Ghosh P, Taylor TK. A longitudinal study of the matrix changes induced in the intervertebral disc by surgical damage to the annulus fibosus. J Orthop Res. 1992; 10:665–676.
6). Moullier P, Bohl D, Heard JM, Danos O. Correction of lysosomal storage in the liver and spleen of MPS VII mice by implantation of genetically modified skin fibroblasts. Nat Genet. 1993; 4:154–159.

7). Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995; 20:1307–1314.

8). Adams MA, May S, Freeman BJ, Morrison HP, Dolan P. Effects of backward bending on lumbar intervertebral discs. Relevance to physical therapy treatments for low back pain. Spine. 2000; 25:431–437.
9). Butler D, Trafimow JH, Andersson GB, McNeill TW, Huckman MS. Discs degenerate before facets. Spine. 1990; 15:111–113.

10). Herkowitz HN, Abraham DJ, Albert TJ. Management of degenerative disc disease above an L5-S1 segment requiring arthrodesis. Spine. 1999; 24:1268–1270.

11). Modic MT. Degenerative disc disease and back pain. Magn Reson Imaging Clin N Am. 1999; 7:481–491.

12). Thompson JP, Oegema TR, Bradford DS. Stimulation of mature canine intervertebral disc by growth facors. Spine. 1991; 16:253–260.
13). Osada R, Ohshima H, Ishihara H. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insuline-like growth factors-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996; 14:690–699.
14). Gruber HE, Fisher EC, Desani B, Stasky AA, Hoelscher G, Hanley EN. Human intervertebral disc cells from the annulus: three dimensional culture in agarose or alginate and responsiveness to TGF-b1. Exp Cell Res. 1997; 235:13–21.
15). Takegami K, Matuda K, Kumano F. Osteogenic pro -tein-1 is most effective in stimulating nucleus pulposus and annulus fibrosus cells to repair their matrix after chon -droitinase ABC-induced chemonucleolysis. 45th Annual Meeting Trans Orthop Res Soc. 1999; 201.
16). Nishimura K, Mochida J. Percutaneous reisertion of the nucleus pulposus: an experimental study. Spine. 1998; 23:1531–1638.
17). Okuma M, Mochida J, Nishimura K, Sakabe K. Nucleus pulposus allograft retards intervertebral disc degeneration: an in vitro and in vivo experimental study. J Orthop Res. 2000; 18:988–997.
18). Evans CH, Robbins PD. Possible orthopaedic applications of gene therapy. J Bone Joint Surg. 1995; 77-A:1103–1114.

19). Evans CH, Robbins PD. Genetically augmented tissue engineering of the musculoskeletal system. Clin Orthop. 1999; 367:S410–8.

20). Evans CH, Robbins PD. Potential treatment of osteoarthritis by gene therapy. Rheum Dis Clin North Am. 1999; 25:333–44.

21). Barr E, Leiden JM. Systemic delivery of recombinant proteins by genetically modified myoblasts. Science. 1991; 4:1507–509.

22). Day CS, Bosch P, Kasemkij WC. Use of muscle cells to mediate gene transfer to the bone defect. Tissue Eng. 1999; 5:119–125.

23). Dhawan J, Pan LC, Pavlath GK, Travis MA, Lanctot AM, Blau HM. Systemic delivery of human growth hor -mone by injection of genetically engineered myoblasts. Science. 1991; 254:1509–1512.
24). Naffakh N, Henri A, Villeval JL. Sustained delivery of erythropoietin in mice by genetically modified skin fibrob -last. Pro Natl Acad Sci. 1995; 92:3194–3198.
25). Caplan AI, Elyanderani M, Mochizuki Y, Wakitani S, Goldberg VM. Principles of cartilage repair and regeration. Clin Ortho Rel Res. 1997; 342:254–269.
26). Haynesworth SE, Goshima J, Goldberg VM, Caplan AI. Characterization of cells with osteogenic potential from human marrow. Bone. 1992; 13:81–88.

27). Haynesworth SE, Reuben D, Caplan AI. Cell-based tissue engineering therapies: the influence of whole body physiology. Adv Drug Del Rev. 1998; 33:3–14.

28). Quintavalla J, Uziel-Fusi S, Yin J, Boehnlein E, Pastor G, Blancuzzi V, Singh HN, Kraus KH, O’ Byrne E, Pelles TC. Fluorescently labeled mesenchymal stem cells(MSCs) maintain multilineage potential and can be detected following implantation into articular cartilage defects. Biomaterials. 2002; 23:109–119.
29). Wakitani S, Goto T, Pineda S J, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg. 1994; 76A:579–592.

30). Im G-I, Kim D-Y, Shin J-H, Hyun C-W, Cho W-H. Re pair of cartilage defect in the rabbit with cultured mesenchymal stem cells from bone marrow. J Bone Joint Surg. 2001; 83B:289–294.
31). Moon S-H, Kang JD, Nishida K, Gilberson LG, Niy-ibizi C, Smith PN, Knaub MA, Robbins PD, Evans CH. Human cervicl intervertebral disc cells are susceptible to adenovirus-mediated gene therapy. Proc Cerv Spine Res Soc. 1999; 150-153.
32). Moon S-H, Nishida K, Kang JD, Gilberson LG, Muz-zonigro TS, Knaub MA, Robbins PD, Evans CH. Human intervertebral disc cells are genetically modifiable in vitro by adenovirus-mediated gene transfer: Implications for the clinical management of intervertebral disc disorder. Spine. 2000; 25:2573–2579.
33). Nishida K, Kang JD, Gilberson LG, Moon S-H, Suh J-K, Vogt MT, Robbins PD, Evans CH. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: An in vivo study of adenovirus-mediated transfer of the human transforming growth factor β1 encoding gene. Spine. 1999; 24:2419–2425.
34). Nishida K, Kang JD, Suh J-K, Robbins PD, Evans CH, Gilberson LG. Adenovirus-mediated gene transfer to nucleus pulposus cells: Implication for the treatment of intervertebral disc degeneration. Spine. 1998; 23:2437–2443.
35). Kim D-J, Moon S-H, Kim H, Kwon U-H, Park M-S, Han K-J, Hahn S-B, Lee H-M. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine. 2003; 28:2679–2684.

36). Hanada K, Solchaga LA, Caplan AI, Hering TM, Goldberg VM, Yoo JU, and Johnstone B. BMP-2 induction and TGF-β1 modulation of rat periosteal cell chon -drogenesis. J Cell Biochem. 2001; 81:284–294.
Go to : 
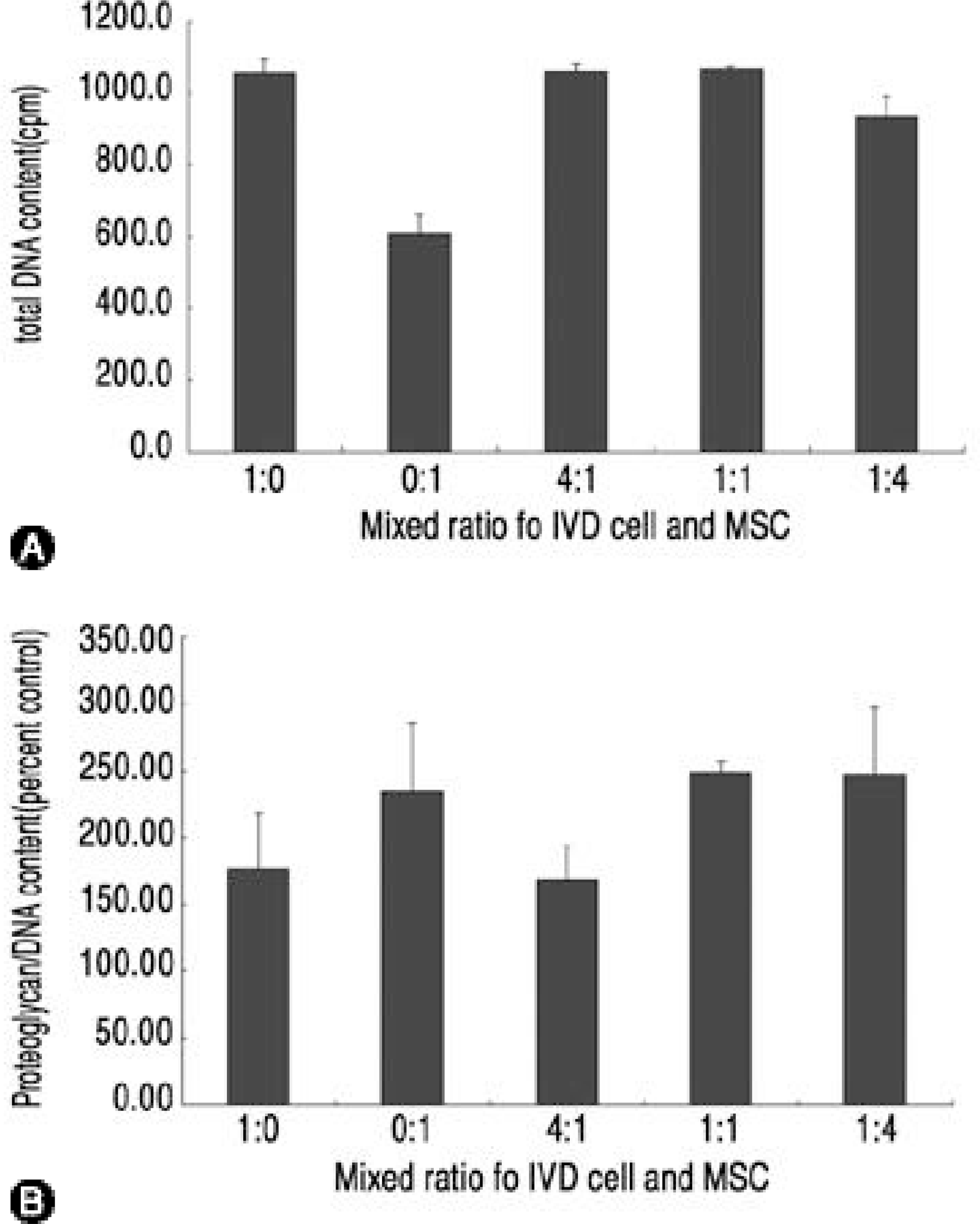 | Fig. 1.(A) total DNA content in three dimensional culture of IVD cells and MSCs, (B)Newly synthesized proteoglycan in three dimensional culture of IVD cells and MSCs presented as percent control. Mixed cultures with IVD cells and MSCs equalled or quite decreased in newly synthesized proteoglycan comparing only IVD cell cultures (p 〈0.05). |
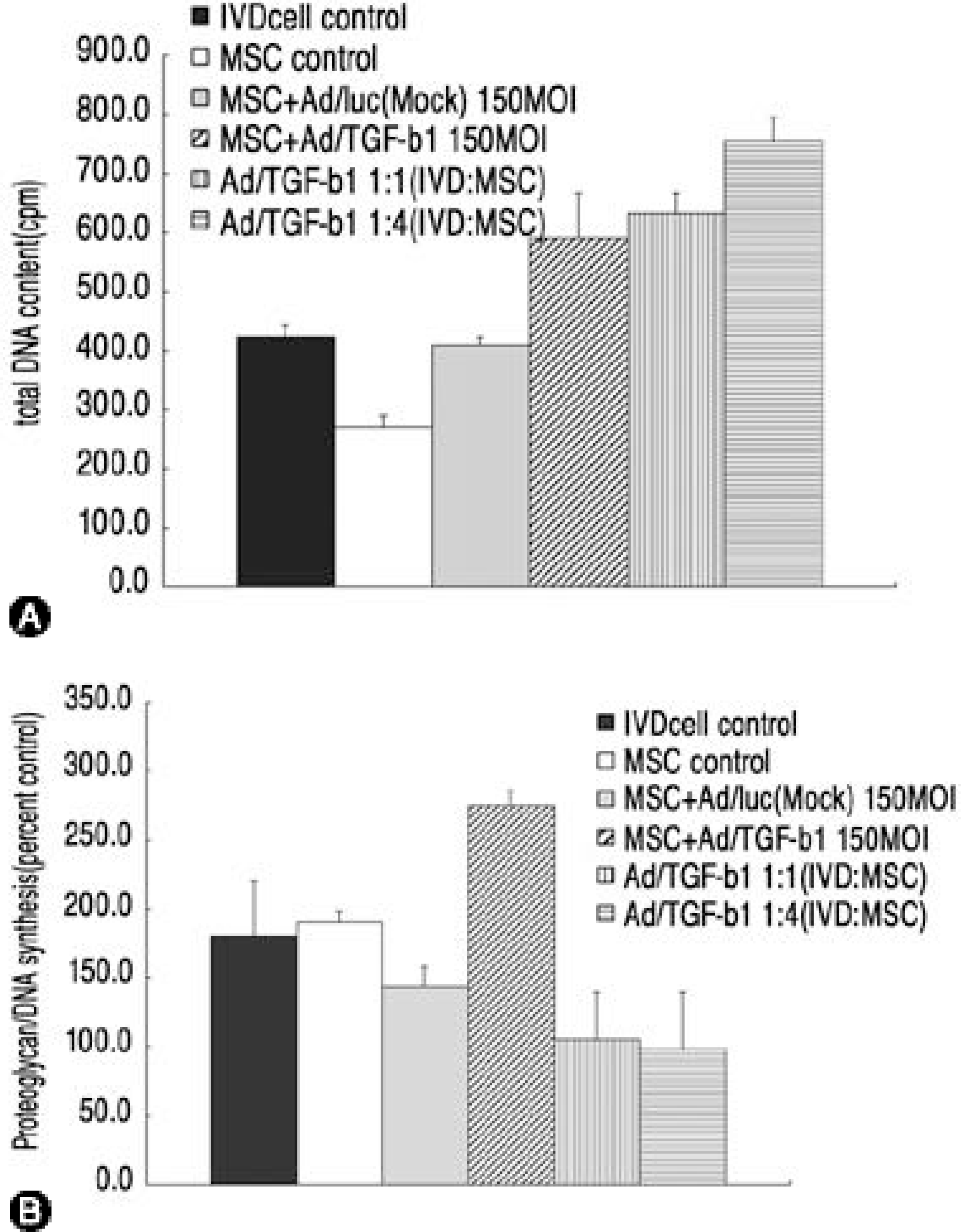 | Fig. 2.(A) total DNA content in three dimensional culture of IVD cells and Ad/TGF-β1-transduced MSCs, (B) Newly synthesized proteoglycan in three dimensional culture of IVD cells and Ad/TGF-β1-transduced MSCs presented as percent control (p 〈0. 05). |
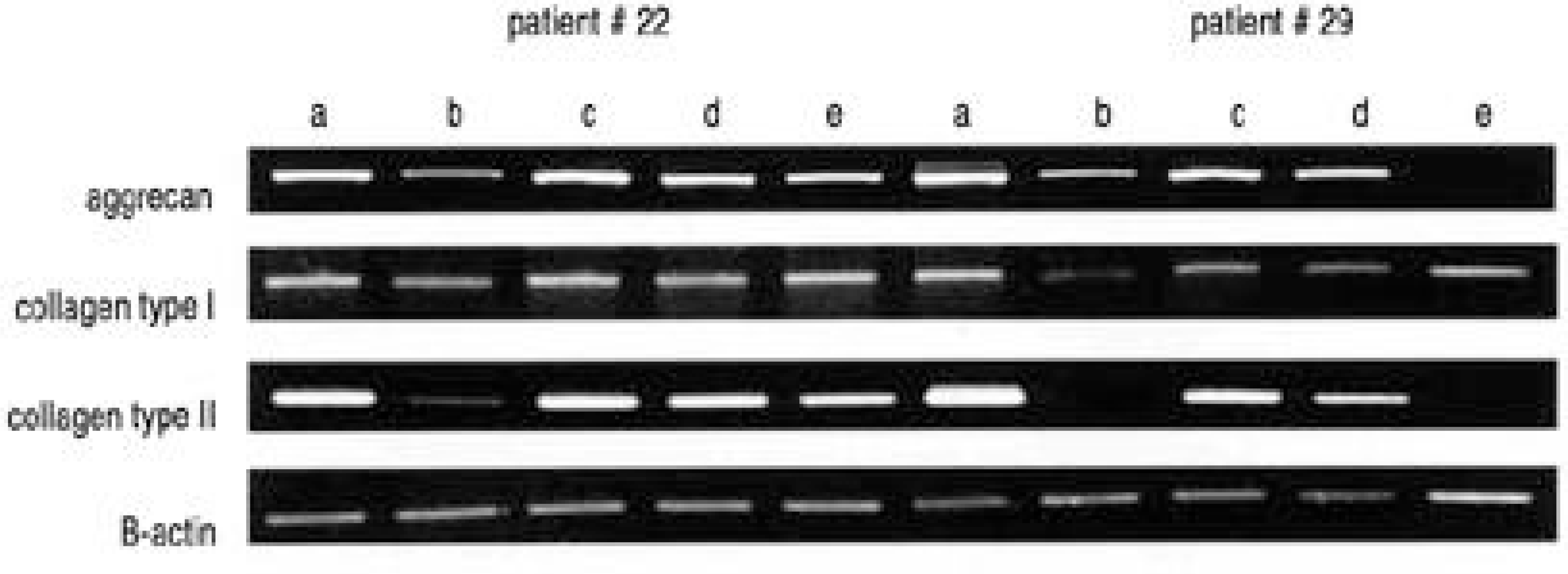 | Fig. 3.RT-PCR for aggrecan, collagen type I, and collagen type II in three dimensional culture of IVD cells and MSCs. β-actin was used for normalization. Mixed ratio of IVD cells and MSCs are a) 1:0, b) 0:1, c)4:1, d) 1:1, e) 1:4. |
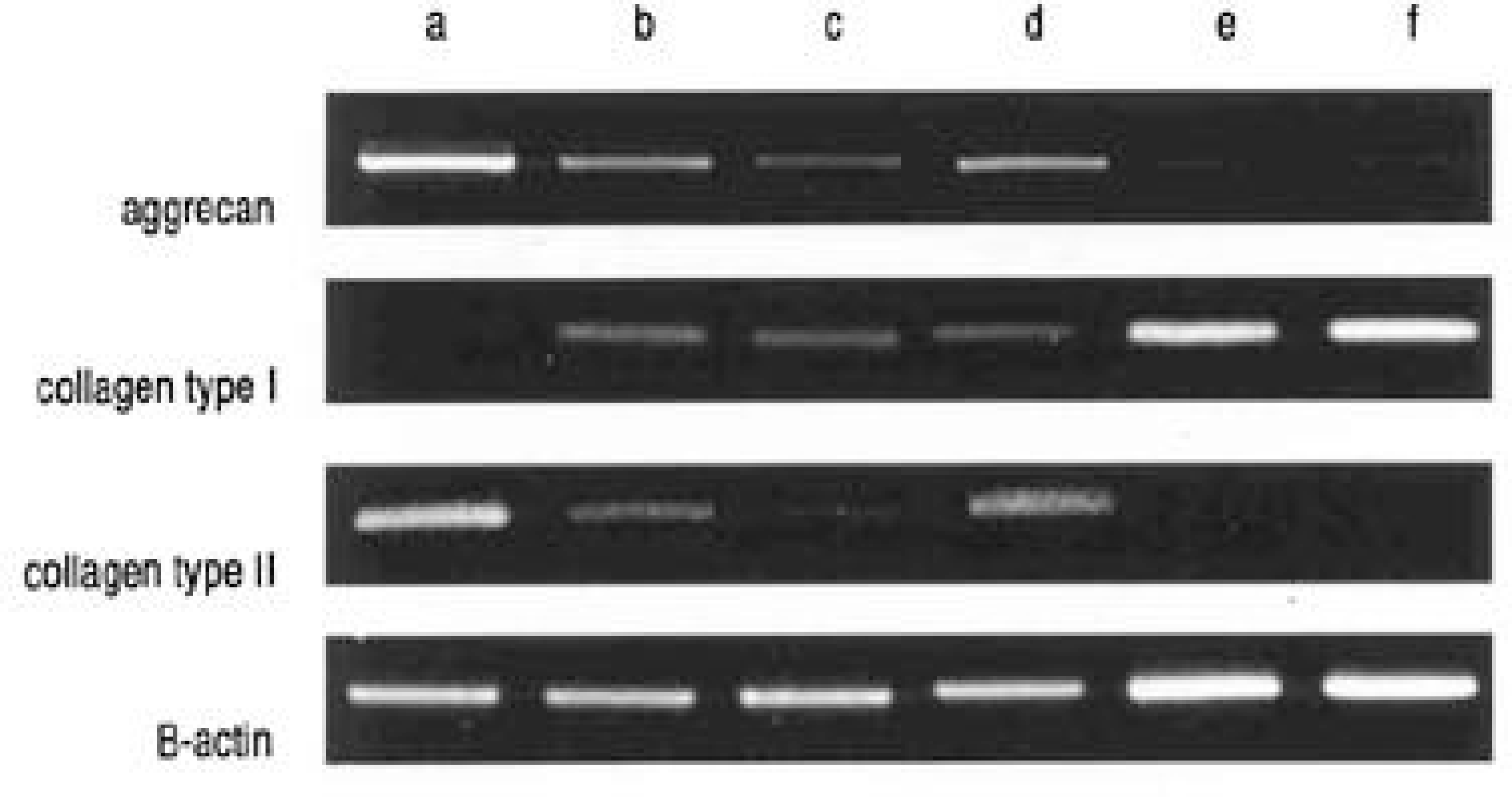 | Fig. 4.RT-PCR for aggrecan, collagen type I, and collagen type II in three dimensional culture of IVD cells and Ad-transduced MSCs. β-actin was used for normalization. a) only IVD cells, b) only MSC, c) only Ad/luc-transduced MSC [Mock], d) only Ad/TGF-β1-transduced MSC [Ad/TGF-β1-MSC], e) [IVD cells : Ad/TGF-β1-MSC] 1:1, f) [IVD cells : Ad/TGF-β1-MSC] 1:4. |
Table 1.
Primer sequence and reaction temperature




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download