Abstract
Objectives
To compare the results of various methodologies for an annulotomy, and evaluate the relationship between the degenerative change and the volume of an extruded nucleus according to the various methodologies.
Summary of Literature Review
Many authors have reported that the traditional annulotomy technique is an open annulotomy.
Material and Methods
16 female white New Zealand rabbits, each weighing about 4- 4.5 kg, were used. A retroperitoneal approach, using a paravertebral incision, was used. Using a number 11 blade, a 21G needle and a number 15 blade, transverse stab incisions, or puncture wounds, were made into the L3- 4, L4- 5 and L5- 6 disc through the antero- lateral annulus. A t all the experimental levels, a complete annulotomy, which confirms the leakage of nucleus pulposus after an annulotomy, was used. To check the extruded nucleus volume after each annulotomy, the gross findings and histological findings of 10 disc samples from each level were analyzed. Radiological methods were used simple lumbar x- ray and MRI. From simple x- rays, the change in the disc height was measured with NIH image software, and the degeneration grade was classified using MRI.
Results
The gross and histological findings showed the most advanced degeneration in the number 15 blade annulotomy group (L5- 6 level), with the simple radiographs showing a fast decrease in the disc height. From the MRI findings, early degenerative findings were observed 2 months after the annulotomy in the number 11 and 15 blades groups. The largest extruded nucleus volume was observed in the number 15 blade annulotomy group.
Go to : 
REFERENCES
1). Bao Q-B., McCullen GM., Higham PA., Dumbleton JH., Yuan HA. Review-The artificial disc: theory, design and materials. Biomaterials. 17:1157–1167. 1996.
2). Bradford DS., Oegema TR., Cooper KM., Wakano K., Chao EY. Chymopapain, chemonucleolysis, and nucleus pulposus regeneration. A biochemical and biome -chanical study. Spine. 9:135–147. 1984.
3). Brown MD. Update on chemonucleolysis. Spine. 21-24S:62S–68S. 1996.
4). Hutton WC., Ganey TM., Elmer WA., Kozlowska E., Ugbo JL., Doh E-S., Whitesides Jr TE. Does long-term compressive loading on the intervertebral disc cause degeneration? Spine. 25:2993–3004. 2000.

5). Iatridis JC., Mente PL., Stokes IA., Aronsson DD., Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine. 24:996–1002. 1999.

6). Klara PM., Ray CD. Artificial nucleus replacement. Clinical experience. Spine. 27:1374–1377. 2002.
7). Lipson SJ., Muir H. Proteoglycans in experimental intervertebral disc degeneration. Spine. 6:194–210. 1981.

8). Nishida K., Gilbertson LG., Robbins PD., Evans C., Kang JD. Potential applications of gene therapy to the treatment of intervertebral disc disorders. Clin Orthop,. 379S:234–241. 2000.

9). Osti OL., Vernon-Roberts B. Anulus tears and inter -vertebral disc degeneration. An experimental study using an animal model. Spine, 15-. 8:762–767. 1990.
10). Otani K., Arai I., Mao G-P., Konno S., Olmarker K., Kikuchi S. Experimental disc herniation. Evaluation of the natural course. Spine,. 22:2894–2899. 1997.
11). Pfirrmann CW., Metzdorf A., Zanetti M., Hodler J., Boos N. Magnetic resonance classification of lumbar interbertebral disc degeneration. Spine,. 26:1873–1878. 2001.
12). Smith JW., Walmsley R. Experimental incision of the intervertebral disc. J Bone Joint Surg.,. 33B:612–625. 1951.

Go to : 
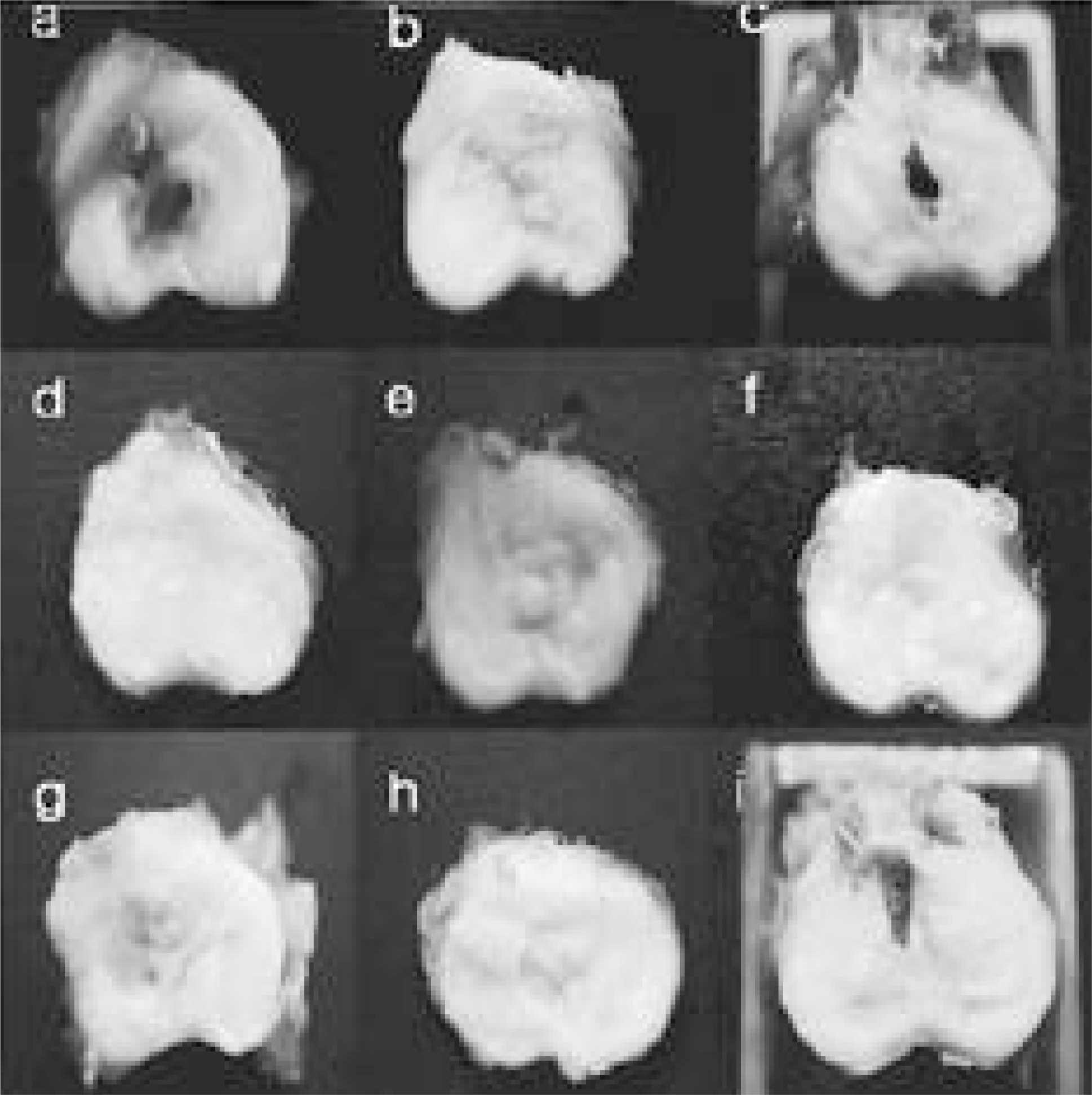 | Fig. 2.In gross findings, it showed the findings of progressive degeneration in all specimens. In 3 month sample (b, h) of 11, 15 blade group, it showed more degeneration than the 21 gauge needle group. It showed severe degeneration in the 15 blade annulotomy 6 month group (i). (No.11 blade annulotmy group is a, b, c. and 21 gauge needle group is d, e, f and 15 blade group is g, h, i. a, d, g is 1 month sample after annulotomy and b, e, h is 3 month sample and c, f, i is 6 month sample.) |
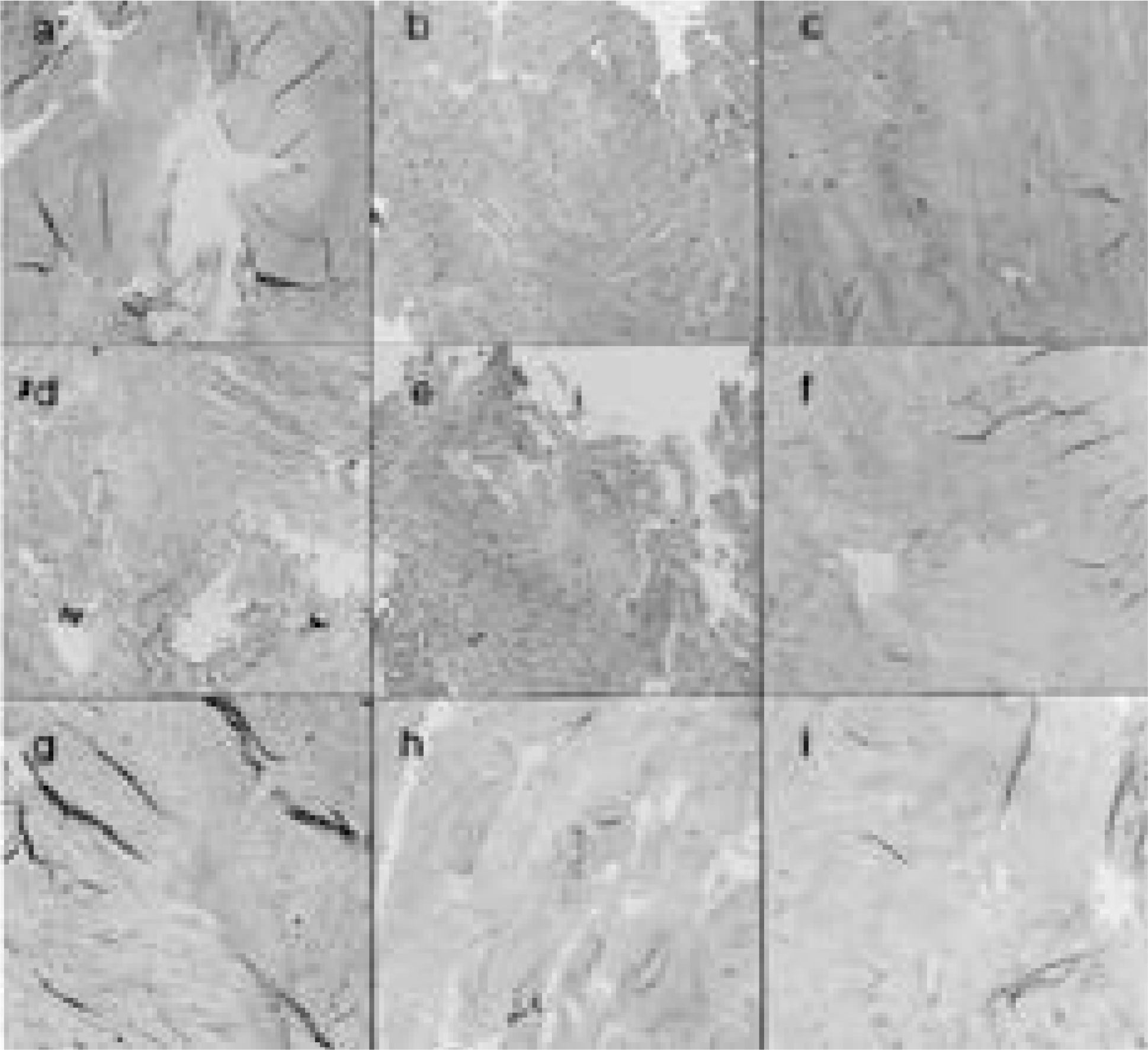 | Fig. 3.Histologic findings showed similar result with gross samples (No. 11 blade annulotmy group is a, b, c. and 21 gauge needle group is d, e, f and 15 blade group is g, h, i. a, d, g is 1 month sample after annulotomy and b, e, h is 3 month sample and c, f, i is 6 month sample.) |
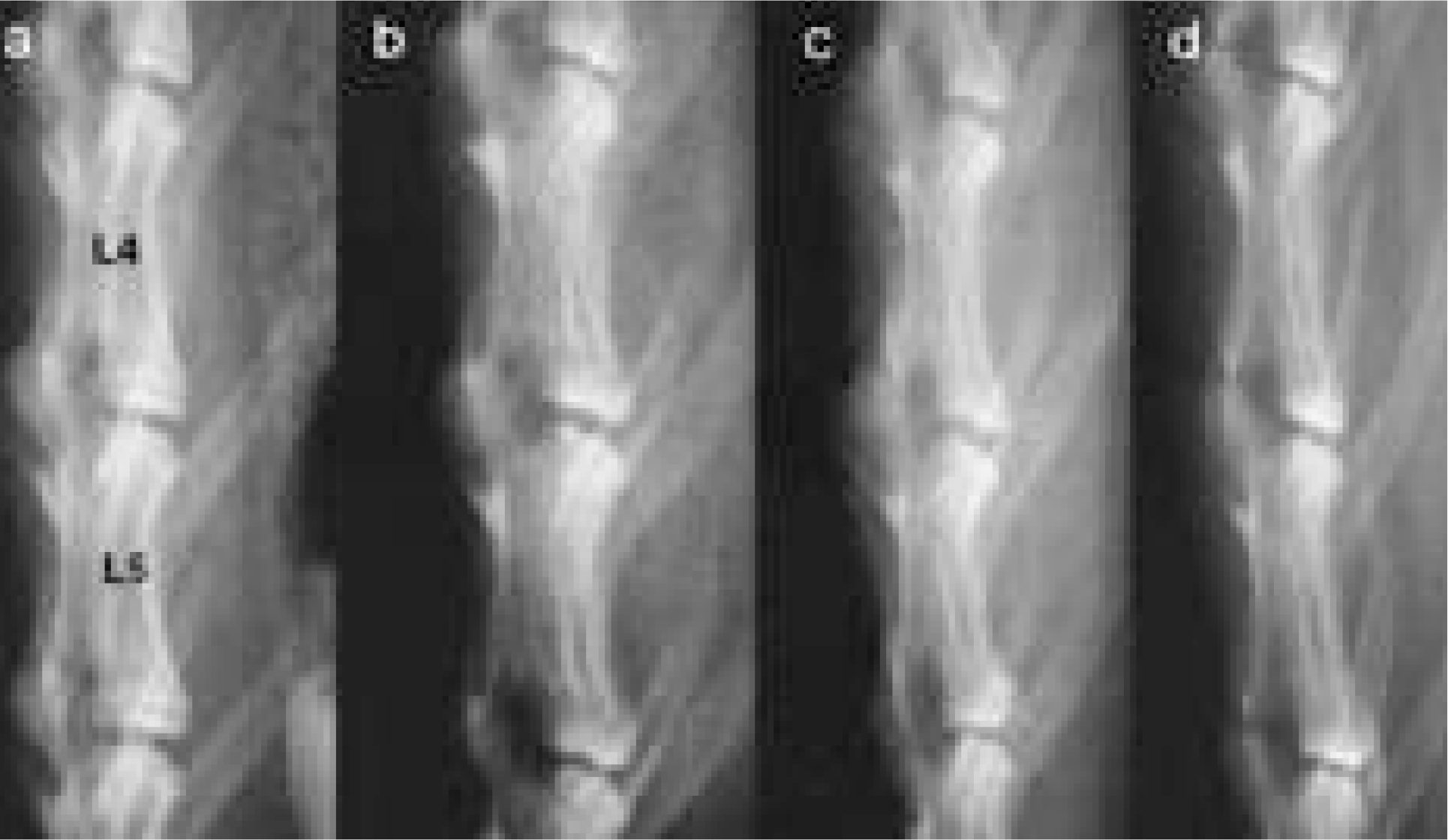 | Fig. 4.The disc height of simple radiography showed progressive narrowing of all experimented disc space. (a is pre-operative film, b is 1 month film, c is 3 month film, and d is 6 month film after annulotomy.) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download