Abstract
Objectives
To evaluate the feasibility of achieving bone formation by percutaneous gene delivery, with plasmid DNA encoding BMP- 7(OP- 1).
Summary of Literature Review
Currently, the preferred method for posterolateral spinal fusion involves decortication of the transverse process, followed by a graft of autogenous bone harvested from the iliac crest. Unfortunately, this procedure suffers from significant morbidity, including blood loss, infection and persistent pain at the harvest site.
Material and Methods
24 Sprague- Dawley rats, weighing approximately 250∼300 g, were used. The percutaneous injection was attempted above both the L5 transverse processes.
The animals were divided into three groups, according to the injection materials: 1) OP- 1 gene/collagen, 2) recombinant OP- 1 protein/collagen and 3) control of PBS/collagen. A t 2 and 4 weeks post- injection, the animals were sacrificed. The gross, radiological and histological findings were analyzed.
Results
No bone was detected grossly by manual palpation or radiography in the groups receiving OP- 1 gene/collagen at either time point. The histological findings revealed the initiation of endochondral bone formation within the paraspinal muscle, directly above the L5 transverse process.
In the rhOP- 1 protein/collagen groups, the gross, radiological and histological findings revealed extensive cartilage and bone formation at both 2 and 4 weeks.
REFERENCES
1). Damien CJ, Parsons JR. Bone graft and bone graft substitutes. A review of current technology applications Journal of Applied Biomaterials. 1991; 2:187–208.
2). Younger EM, Chapman MW. Morbidity at bone graft donor site. J Orthop Trauma. 1989; 3:192–195.
3). Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg. 1989; 71B:677–680.

5). Cook SD, Dalton JE, Tan EH, Whitecloud III TS, Rueger DC. In vivo evaluation of recombinant human osteogenic protein(rhOP-1) implants as a bone graft substitute for spinal fusions. Spine. 1994; 19:1655–1663.
6). Grauer JN, Patel TC, Erulkar JS, Troiano NW, Panjabi MM, Friendlaender GE. Evaluation of op-1 as a graft substitute for intertransverse process lumbar fusion. Spine. 2001; 26:127–133.

7). Salamon ML, Althausen PL, Gupta MC, Laubach J. The effects of BMP-7 in a rat posterolateral intertransverse process fusion model. J Spinal Disord. 2003; 16:90–95.

8). Schimandle JH, Boden SD, Hutton WC. Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine. 1995; 20:1326–1337.

9). Boden SD, Martin GJ, Horton WC, Truss TL, Sandhu HS. Laparoscopic anterior spinal arthrodesis with rh BMP-2 in a titanium interbody threaded cage. J Spinal Disord. 1998; 11:95–101.
10). Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages: Definitive evidence of osteoinduction in humans. Spine. 2000; 25:376–381.
11). Sandhu HS, Kanim LEA, Kabo JM et al.E f fe ct iv e doses of recombinant bone morphogenetic protein-2 in experimental spinal fusion. Spine. 1996; 21:2115–2122.
12). Alden TD, Pittman DD, Beres EJ et al.Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. J. Neurosurg. 1999; 90:109–114.
13). Sandhu HS, Khan SN. Animal models for preclinical assessment of bone morphogenetic proteins in the spine. Spine. 2002; 27:S32–S38.

14). Boden SD. Biology of lumbar spine fusion and use of bone graft substitutes: present, future, and next generation. Tissue Engineering. 2000; 6:383–399.

15). Danko I, Williams P, Herweijer H et al.High expression of naked plasmid DNA in muscles of young rodents. Human Mo ecular Genetics. 1997; 6:1435–1443.
Fig. 1.
In gross and radiographic findings of Op-1 gene and collagen injection group, it doesn’ t show the evidence of bone formation (2 week group after harvest is (A, B) and 4 week group is (C, D).
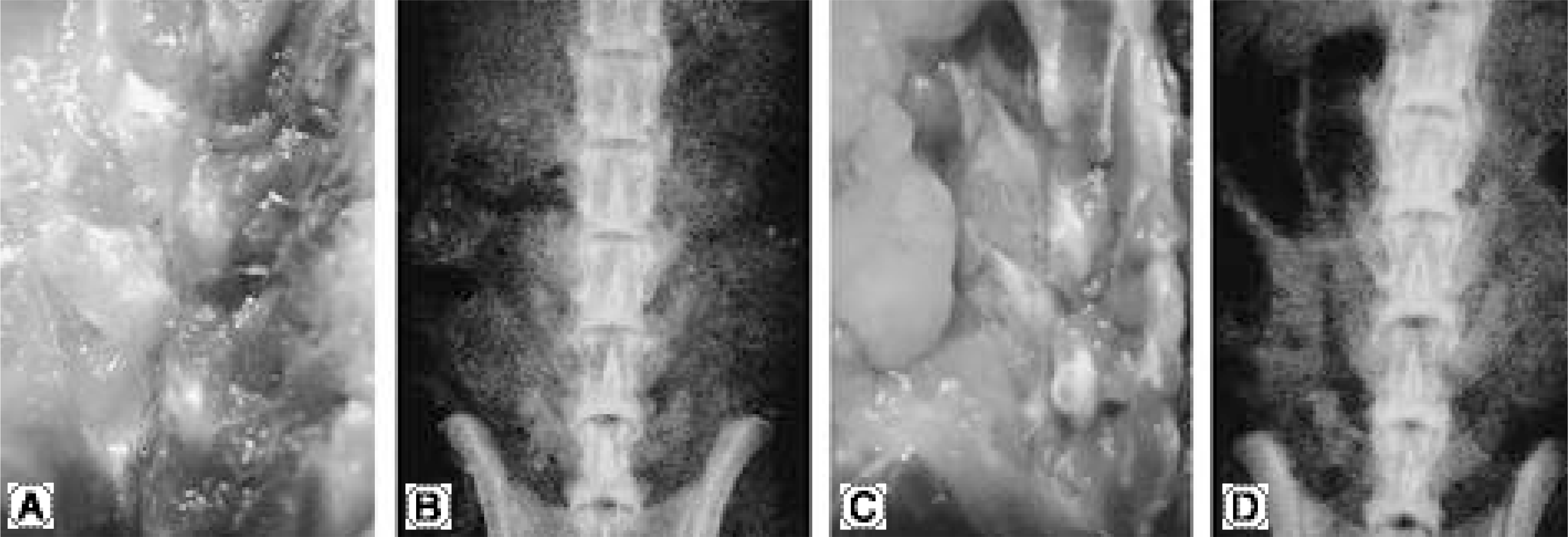
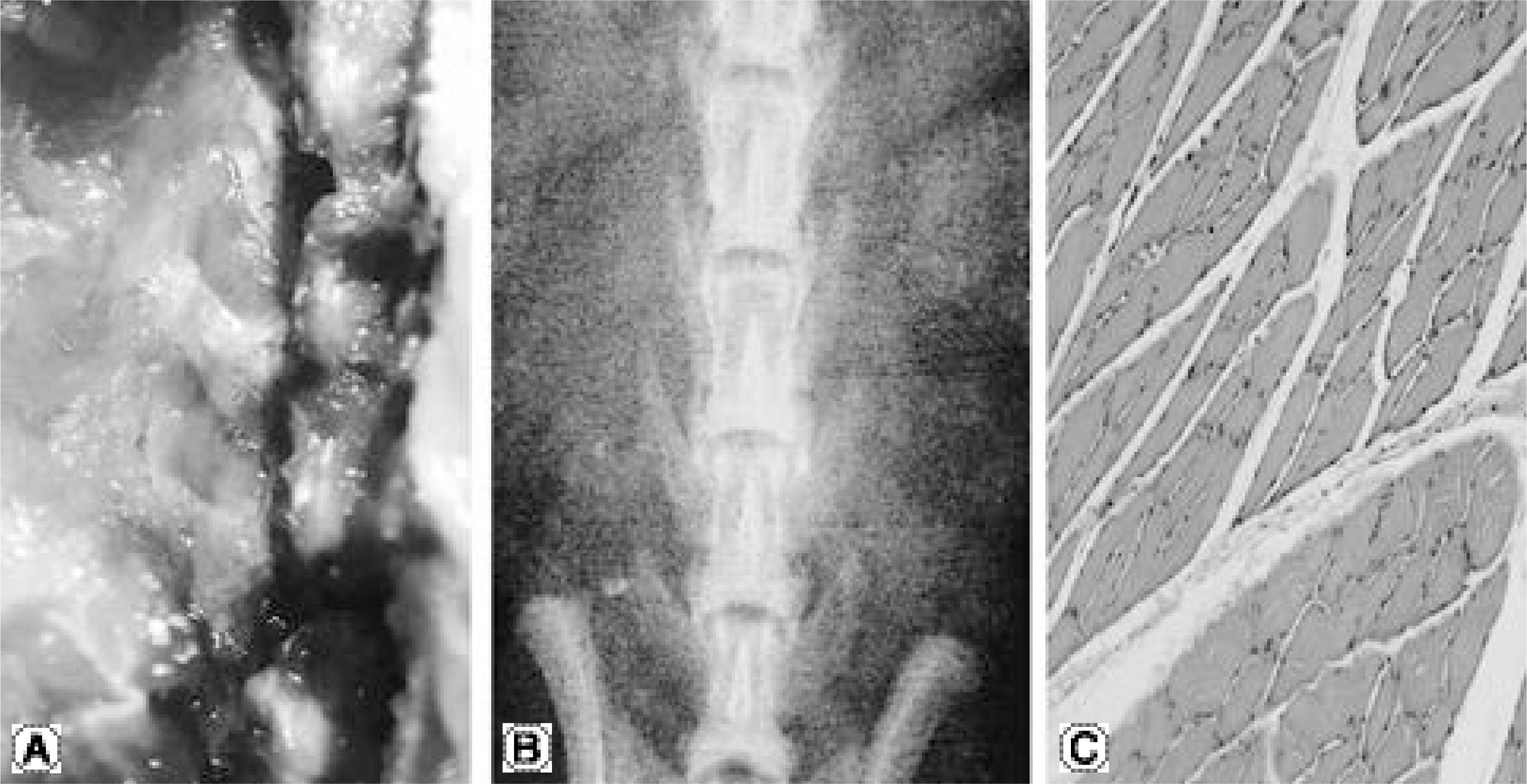
Fig. 2.
Histologic findings of Op-1 gene and collagen injection group. (A) It shows loose cartilaginous matrix and non-woven bone matrix in the dorsal injected surface of transverse process in 2 week histologic findings (H-E staining, × 100). (B) It shows the decrease of granulation tissue and atrophy of the muscle cell around the injection site in 4 week histologic findings (H-E staining, × 200).
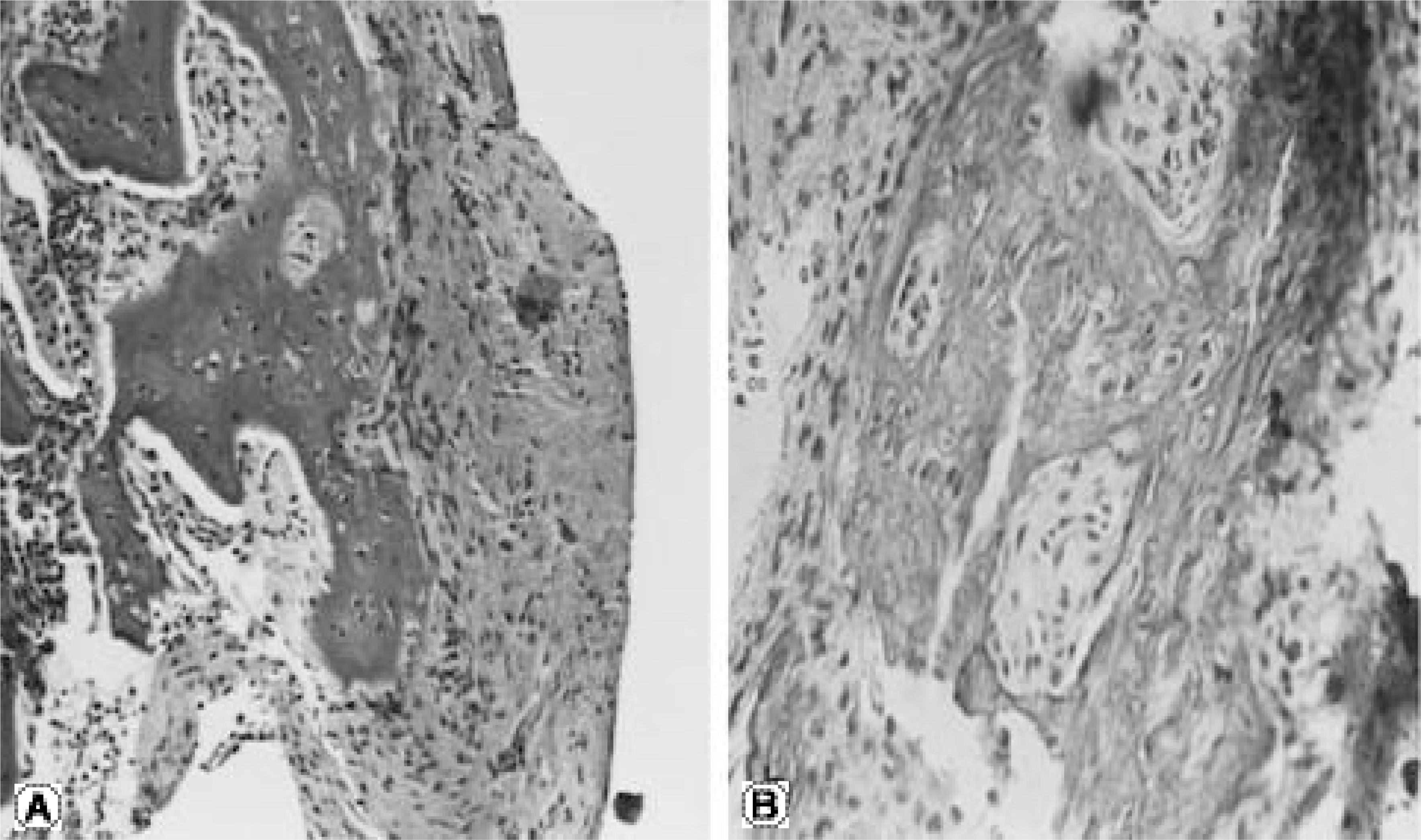
Fig. 3.
In gross and radiographic findings of Op-1 protein and collagen injection group, it shows the evidence of bone formation and firm bony union. 4 week group showed more bone formation than 2 week group. In 4 week group, it shows posterior and facet fusion due to abundant bone formation (2 week group after harvest is A, B and 4 week group is C, D).
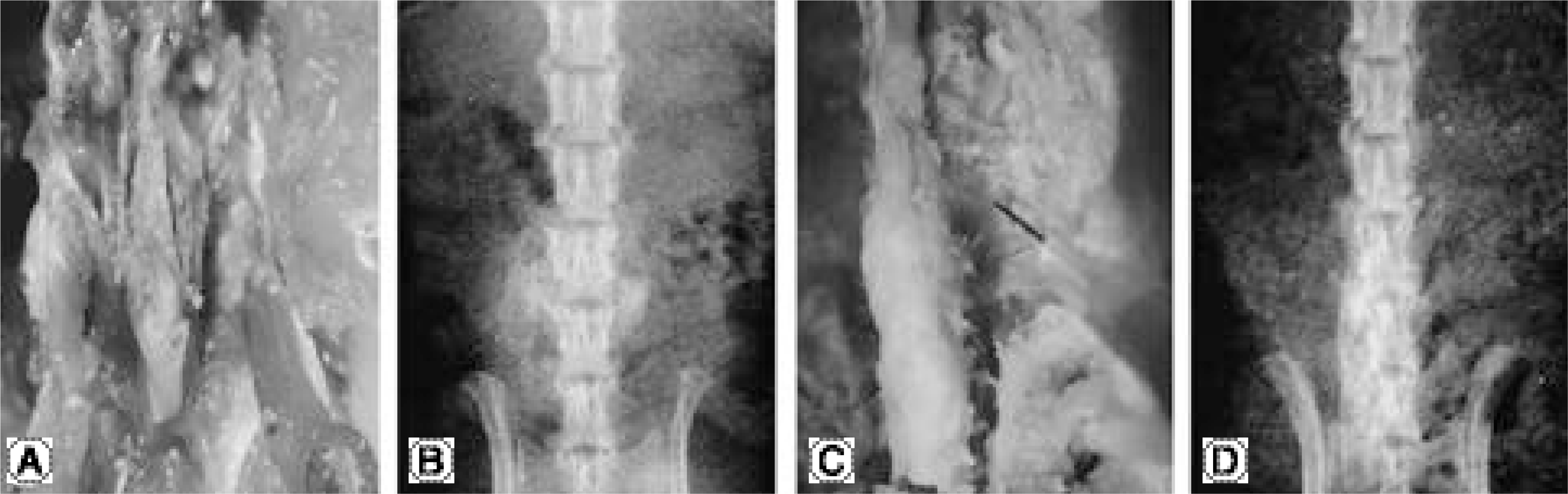
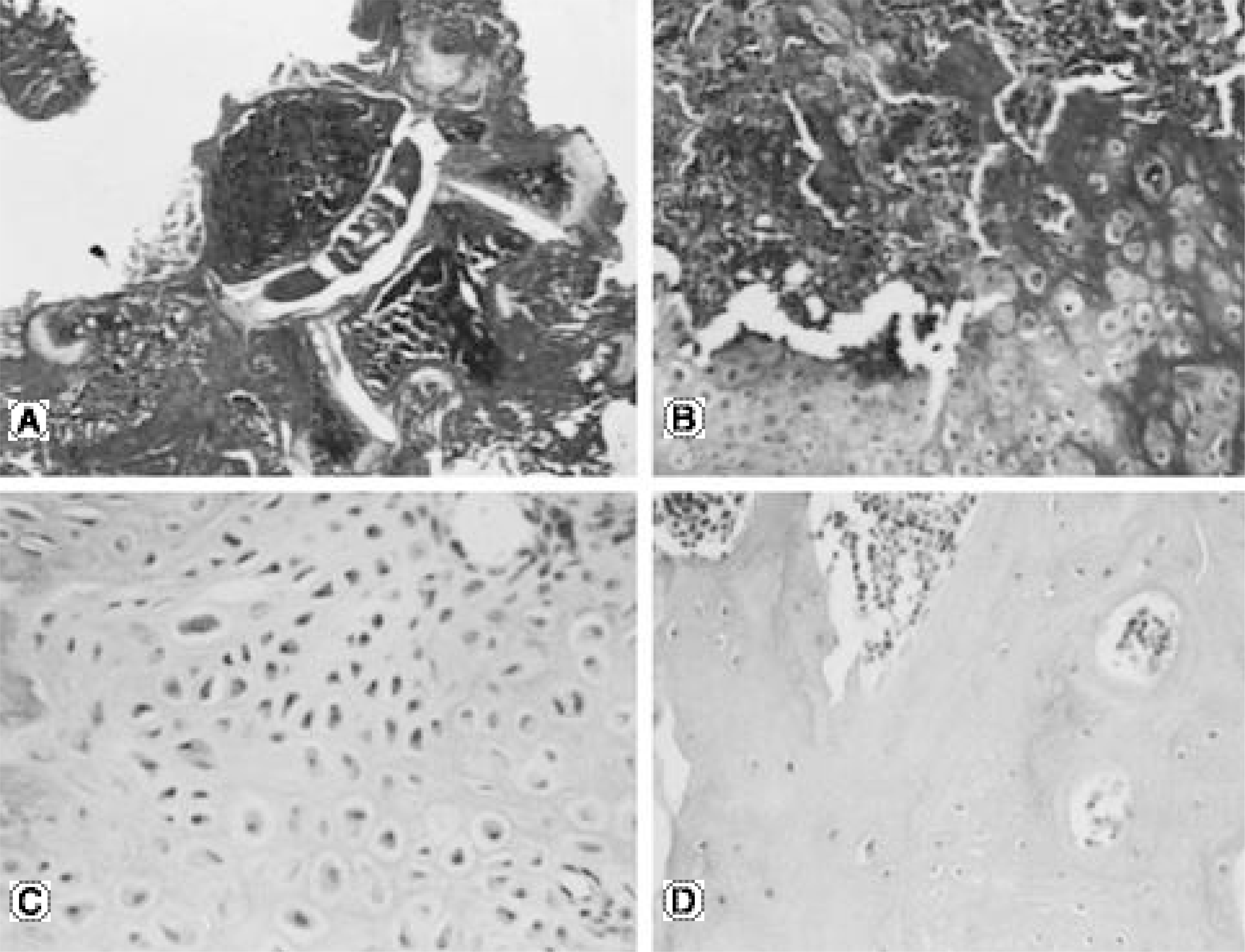
Fig. 4.
Histologic findings of Op-1 protein and collagen injection group. (A, B) It shows the newly formed bone around the posterior aspect of transverse process in 2 week whole spine sample and the definite evidence of endochondral ossification (H-E staining, A: × 10, B: × 100). (C, D) In the intertransverse muscle of 4 week histology, it shows maturation of chondrocyte, mature bone, osteoid and bone marrow (H-E staining, C: × 200, D: × 100).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download