Materials and Methods
Medical records of 375 inpatients admitted in a single center (Kyung Hee University Hospital, Seoul, Korea) who were treated for acute stage of KD from January 2000 to December 2013 were retrospectively reviewed.
The Diagnostic Guidelines for KD (American Heart Association [AHA], 2004) were used as a reference for the diagnostic criteria of KD [
5].
Each patient was treated initially with 2 g/kg intravenous immunoglobulin (IVIG) [
5]. Symptoms and signs were observed for 36 to 48 h after termination of IVIG infusion. Additional IVIG, steroid (especially intravenous methylprednisolone), or infliximab was administered when patients were not responsive to the initial IVIG infusion.
After treatment, all KD patients were followed-up with regular two-dimensional (2D) cardiac echocardiography for one year. Abnormalities of coronary arteries were diagnosed by 2D echocardiography, according to the 2004 AHA guidelines [
5].
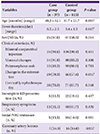
Among 375 patients, 152 (40.5%) patients were also tested for mycoplasma infections by enzyme immunoassay (Serodia-Myco II, Fujirebio, Japan, from 2011). The patients were classified into two groups: 37 (24.3%) with positive results (case group) and 115 (75.7%) with negative results (control group). A positive result was defined as the presence of anti-mycoplasma IgM ≥1:640 or an increase in anti-mycoplasma IgG by more than 4-fold in consecutive tests between 10 to 21 days [
6]. As anti-mycoplasma IgM can remain positive for up to 6 to 12 months after infection, the positive group includes acute and recent infections.
Clinical profiles of the two groups were compared. All laboratory data were collected before IVIG was started. Clinical manifestations, respiratory symptoms, and chest X-rays of the patients with persistent fever after treatment were analyzed.
Positive respiratory symptoms were defined as one or more of the following on admission: cough, sputum, or rhinorrhea.
All statistical analyses were performed using SPSS version 22.0 for Windows (IBM, Chicago, Il, USA). P values of <0.05 were considered as statistically significant. Data were presented as mean ± standard deviation. Continuous numeric variables were analyzed by Student t-test, and categorical variables were analyzed by Pearson Chi-square test or Fisher's exact test.
Discussion
Of the 375 KD patients assessed during the study, 152 patients were tested for mycoplasma, and 37 patients had positive results. Before 2007, the patients were tested for mycoplasma infection in the presence of cough, rhinorrhea or persistent fever. From 2007, when there was a mycoplasma epidemic most patients admitted for KD were initially tested for mycoplasma infections. The group with positive tests had longer fever duration, older age, and more changes in the extremities. Seven (18.9%) patients in the case group had persistent fever even after resolution of the acute stage of KD.
The mean age of KD patients is generally considered to be 3 years; all patients are younger than 5 years [
7]. However, in one study, the age distribution was 9 to 11 months [
3]. Because mycoplasma infection was common in preschool-aged children in our study, the onset age of KD may be slightly higher than in other studies. On the other hand, the youngest mycoplasma-positive child with KD in our study was 2 months of age. Mycoplasma infection in Korea is not limited to school-aged children because many infants and younger children are cared for in daycare centers. Therefore, differential diagnosis between KD and mycoplasma infection is becoming more important in Korea. According to our study, recent mycoplasma infection and KD co-occur more often in older children. Immune modulation of
M. pneumoniae infection may results in cross-reaction between bacterial and human cell components, and autoimmunity may contribute to the pathogenesis of KD [
8].
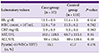
In our study, the mean fever duration was 6.5 days in the mycoplasma-positive group and 5.4 days, in the negative group. In general, fever duration in patients with KD is more than 5 days; it can persist to 14 days when mycoplasma infection present [
9]. Mycoplasma infection alone has a longer fever duration than KD has. In a study by Lee et al. [
8], the mean fever duration was 9.2 days in the mycoplasma-positive group and 6.9 days in the negative group, which was longer than both groups in our study.
In our study, changes in the extremities differed significantly between the mycoplasma-positive and negative groups. The detailed mechanism of the changes of the extremities in the acute stage of KD and the following subacute stage is not well understood. Hand and foot changes in patients with mycoplasma infection have been described in a case report by Greco et al., 2007 [
10]. Although the pathogenesis of the changes has not been fully elucidated, they may be explained by hypersensitivity vasculitis. The patient reported by Greco et al. had extrapulmonary symptoms of
M. pneumoniae infection, namely cutaneous vasculitis, as well as inflammation and necrosis of small blood vessel walls.
Of the 37 case group patients, 7 patients had persistent fever after the resolution of acute symptoms and laboratory abnormalities. They were reevaluated to identify the cause of sustained fever after use of IVIG. Laboratory studies including WBC, Hb, Hct, CRP, AST, ALT and immunology assay were within normal level. Respiratory viral polymerase chain reaction (PCR) was not available for the group. Clarithromycin (CLR), or azithromycin (AZM) was given to the patients with subsequent subsiding of fever.
One of these 7 cases (case 4) is described in
Figure 2. A 5-year-old male presented with all symptoms of acute-stage KD and we started treatment with IVIG on the first hospital day. Almost 48 hours after the end of the first IVIG treatment, fever and erythema of the hands and feet remained. The patient was regarded as having refractory acute stage of KD and the second IVIG treatment was infused on day 4. On day 8, desquamation of hands and feet was observed yet fever persisted. During evaluation for the sustained fever, CRP was decreased and other laboratory findings were normalized, however anti-mycoplasma IgM titer remained high (>1:1,280) until day 10. Azithromycin was administered and fever subsided on day 14. Initially, the child had no respiratory symptoms and showed unremarkable chest X-ray findings. A follow-up chest X-ray was also unremarkable. Therefore, mycoplasma infection may be a cause of persistent fever after the resolution of acute-stage KD.
 | Figure 2
Clinical course of case No. 4 (see Table 3) IVIG, intravenous immunoglobulin; HD, hospital day; BT, body temperature; CRP, c-reactive protein

|
In our study, 11 (29.7%) of the 37 case group patients were treated with macrolide antibiotics. Antibiotics were not used in the other patients.
Mycoplasma is known as a main cause of pneumonia in school-aged children. Our results seem to indicate high incidence of recent mycoplasma infection in preschool-aged children. Mycoplasma infection has many extrapulmonary symptoms such as arthritis, hemolytic anemia and other systemic symptoms [
11]. Symptoms of mycoplasma infection may be similar to those of KD and concomitant infection with mycoplasma in KD patients is also possible. Therefore, it is important to distinguish recent mycoplasma infection from KD in patients with fever that persists despite appropriate management of KD.
A study revealed some factors which predict refractory KD: male gender, cervical lymphadenopathy, decreased platelet count, changes of extremities, increased total bilirubin, increased alkaline phosphatase (ALP), elevated lactate dehydrogenase(LDH), and elevated CRP [
12]. In another study by Kim et al. [
13], CRP, AST, ALT, and the neutrophil differential were higher while the lymphocyte differential, protein, albumin, platelet count, and total cholesterol were decreased in the refractory group.
IVIG with conventional antibiotics in early treatment of macrolide-resistant mycoplasma pneumonia can prevent disease progression. In 4 patients with macrolide-resistant mycoplasma pneumonia in the study by Youn et al. [
14], corticosteroid was previously used for immune modulation. In those patients with remaining pneumonia, IVIG treatment improved the lesions within 2 days.
There are a few limitations in our study. Some laboratory findings, for example, urine WBC, had either positive or negative categorical values. Early diagnosis of mycoplasma infection was challenging, especially in its acute stage, because of a low antibody level. In addition, respiratory virus RT-PCR testing was not performed for all patients in this study.
In this study, most patients admitted for treatment of KD from 2007 were initially tested for possible mycoplasma infection. Cross-reactivities of IVIG and anti-mycoplasma antibody may be considered in patients who were admitted before 2007. The false-positive IgM ratio of enzyme immunoassay methods vary from 2.8 - 14.9% [
15]. When PCR is combined with the immunoassay method, the sensitivity of rapid diagnosis is 95% [
16].
In conclusion, recent mycoplasma infection was somewhat related to KD. The mean age of the mycoplasma-positive group was 48.2 months, which was significantly older than the negative group.
When fever persists after the resolution of acute symptoms and laboratory abnormalities of KD, further evaluation and treatment for mycoplasma infection may be considered, especially in preschool-aged children.






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download