1. Ramanan P, Razonable RR. Cytomegalovirus infections in solid organ transplantation: a review. Infect Chemother. 2013; 45:260–271.

2. Browne BJ, Young JA, Dunn TB, Matas AJ. The impact of cytomegalovirus infection ≥1 year after primary renal transplantation. Clin Transplant. 2010; 24:572–577.

3. Cordero E, Casasola C, Ecarma R, Danguilan R. Cytomegalovirus disease in kidney transplant recipients: incidence, clinical profile, and risk factors. Transplant Proc. 2012; 44:694–700.

4. Giakoustidis D, Antoniadis A, Fouzas I, Sklavos A, Giakoustidis A, Ouzounidis N, Gakis D, Koubanagiti K, Myserlis G, Tsitlakidis A, Gerogiannis I, Papagiannis A, Christoforou P, Deligiannidis T, Solonaki F, Imvrios G, Papanikolaou V. Prevalence and clinical impact of cytomegalovirus infection and disease in renal transplantation: ten years of experience in a single center. Transplant Proc. 2012; 44:2715–2717.

5. Hughes D, Hafferty J, Fulton L, Friend P, Devaney A, Loke J, Welsh KI, Handa A, Klenerman P. Donor and recipient CMV serostatus and antigenemia after renal transplantation: an analysis of 486 patients. J Clin Virol. 2008; 41:92–95.

6. Jung GO, Kim SJ, Choi GS, Moon JI, Kim JM, Sin MJ, Kim EY, Kwon CH, Joh JW, Lee SK. The effect of cytomegalovirus antigenemia titer on the efficacy of preemptive therapy for the prevention of cytomegalovirus disease after kidney transplantation. Transplant Proc. 2010; 42:804–810.

7. De Keyzer K, Van Laecke S, Peeters P, Vanholder R. Human cytomegalovirus and kidney transplantation: a clinician's update. Am J Kidney Dis. 2011; 58:118–126.

8. Selvey LA, Lim WH, Boan P, Swaminathan R, Slimings C, Harrison AE, Chakera A. Cytomegalovirus viraemia and mortality in renal transplant recipients in the era of antiviral prophylaxis. Lessons from the western Australian experience. BMC Infect Dis. 2017; 17:501.

9. Andrews PA, Emery VC, Newstead C. Summary of the British transplantation society guidelines for the prevention and management of CMV disease after solid organ transplantation. Transplant. 2011; 92:1181–1187.

10. Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Snydman DR, Allen U, Humar A; Transplantation Society International CMV Consensus Group. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplant. 2010; 89:779–795.

11. Fisher CE, Knudsen JL, Lease ED, Jerome KR, Rakita RM, Boeckh M, Limaye AP. Risk factors and outcomes of ganciclovir resistant cytomegalovirus infection in solid organ transplant recipients. Clin Infect Dis. 2017; 65:57–63.

12. Kowalsky S, Arnon R, Posada R. Prevention of cytomegalovirus following solid organ transplantation: a literature review. Pediatr Transplant. 2013; 17:499–509.

13. Saracino A, Colucci R, Latorraca A, Muscaridola N, Procida C, Di Noia I, Santospirito VE, Santarsia G. The effects of preemptive therapy using a very low threshold of pp65 antigenemia to prevent cytomegalovirus disease in kidney transplant recipients: a single-center experience. Transplant Proc. 2013; 45:182–184.

14. Atkinson C, Emery VC. Cytomegalovirus quantification: where to next in optimising patient management? J Clin Virol. 2011; 51:223–228.

15. Razonable RR, Hayden RT. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation. Clin Microbiol Rev. 2013; 26:703–727.

16. Ljungman P, Boeckh M, Hirsch HH, Josephson F, Lundgren J, Nichols G, Pikis A, Razonable RR, Miller V, Griffiths PD; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017; 64:87–91.

17. Jamal AJ, Husain S, Li Y, Famure O, Kim SJ. Risk factors for late-onset cytomegalovirus infection or disease in kidney transplant recipients. Transplant. 2014; 97:569–575.

18. Harvala H, Stewart C, Muller K, Burns S, Marson L, MacGilchrist A, Johannessen I. High risk of cytomegalovirus infection following solid organ transplantation despite prophylactic therapy. J Med Virol. 2013; 85:893–898.

19. Schröeder R, Michelon T, Fagundes I, Bortolotto A, Lammerhirt E, Oliveira J, Santos A, Bittar A, Keitel E, Garcia V, Neumann J, Saitovitch D. Antigenemia for cytomegalovirus in renal transplantation: choosing a cutoff for the diagnosis criteria in cytomegalovirus disease. Transplant Proc. 2005; 37:2781–2783.

20. Pilmore H, Pussell B, Goodman D. KHA-CARI guideline: cytomegalovirus disease and kidney transplantation. Nephrol. 2011; 16:683–687.


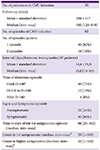
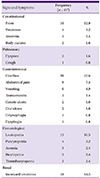




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download