Introduction
Case Report
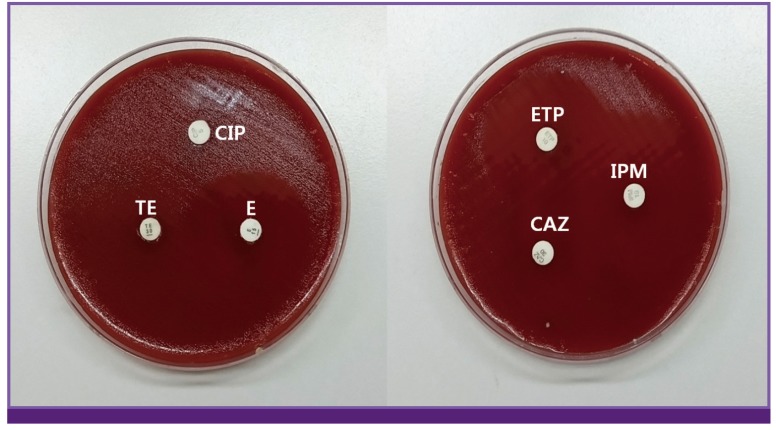 | Figure 2Antibiotic susceptibility test for isolated Campylobacter jejuni by disk diffusion method.
CIP, ciprofloxacin; TE, tetracyclin; E, erythromycin; ETP, ertapenem; IPM, imipenem; CAZ, ceftazidime.
|
Table 1
Antibiotic susceptibility of isolated Campylobacter jejuni: disk diffusion method standardized and checked by European Committee on Antimicrobial Susceptibility Testing (EUCAST)
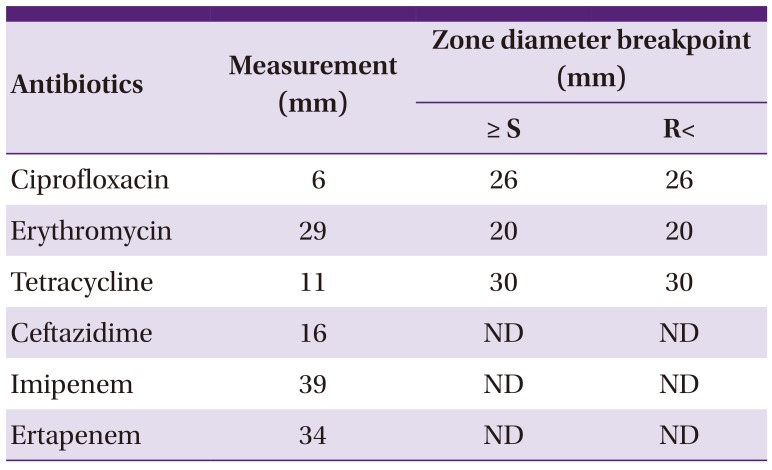
| Antibiotics | Measurement (mm) | Zone diameter breakpoint (mm) | |
|---|---|---|---|
| ≥ S | R< | ||
| Ciprofloxacin | 6 | 26 | 26 |
| Erythromycin | 29 | 20 | 20 |
| Tetracycline | 11 | 30 | 30 |
| Ceftazidime | 16 | ND | ND |
| Imipenem | 39 | ND | ND |
| Ertapenem | 34 | ND | ND |
Discussion
Table 2
Reported Campylobacter bacteremia cases in Asian countries during 2000-2014
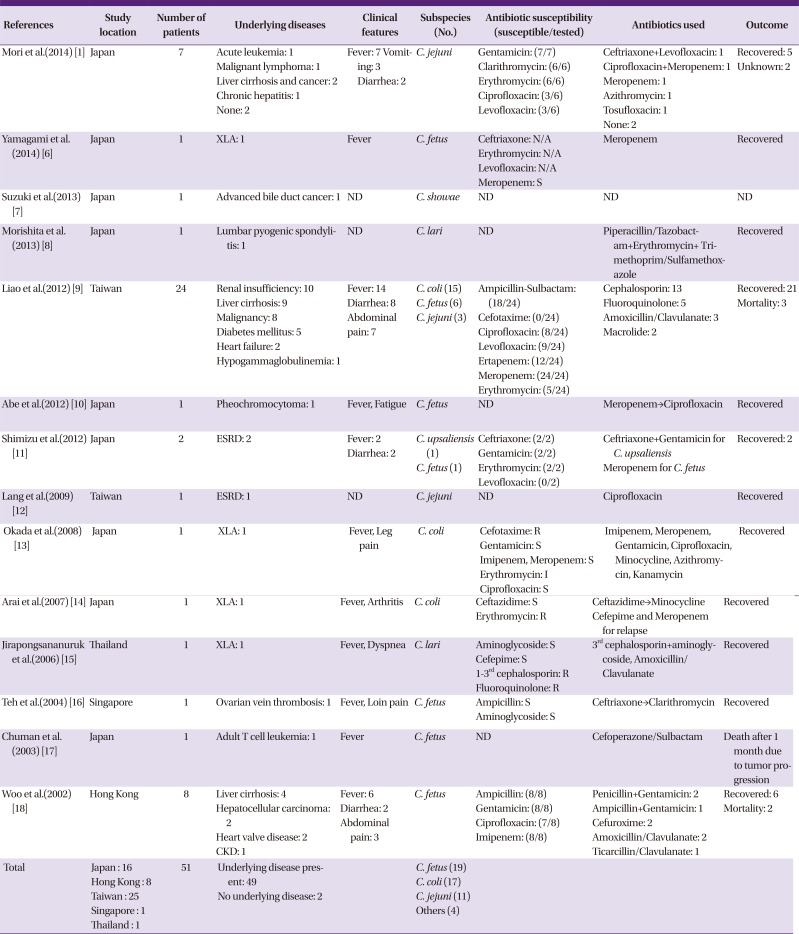
| References | Study location | Number of patients | Underlying diseases | Clinical features | Subspecies (No.) | Antibiotic susceptibility (susceptible/tested) | Antibiotics used | Outcome |
|---|---|---|---|---|---|---|---|---|
| Mori et al.(2014) [1] | Japan | 7 |
Acute leukemia: 1 Malignant lymphoma: 1 Liver cirrhosis and cancer: 2 Chronic hepatitis: 1 None: 2 |
Fever: 7 Vomiting: 3 Diarrhea: 2 |
C. jejuni |
Gentamicin: (7/7) Clarithromycin: (6/6) Erythromycin: (6/6) Ciprofloxacin: (3/6) Levofloxacin: (3/6) |
Ceftriaxone+Levofloxacin: 1 Ciprofloxacin+Meropenem: 1 Meropenem: 1 Azithromycin: 1 Tosufloxacin: 1 None: 2 |
Recovered: 5 Unknown: 2 |
| Yamagami et al.(2014) [6] | Japan | 1 | XLA: 1 | Fever | C. fetus |
Ceftriaxone: N/A Erythromycin: N/A Levofloxacin: N/A Meropenem: S |
Meropenem | Recovered |
| Suzuki et al.(2013) [7] | Japan | 1 | Advanced bile duct cancer: 1 | ND | C. showae | ND | ND | ND |
| Morishita et al.(2013) [8] | Japan | 1 | Lumbar pyogenic spondylitis: 1 | ND | C. lari | ND | Piperacillin/Tazobactam+Erythromycin+Trimethoprim/Sulfamethoxazole | Recovered |
| Liao et al.(2012) [9] | Taiwan | 24 |
Renal insufficiency: 10 Liver cirrhosis: 9 Malignancy: 8 Diabetes mellitus: 5 Heart failure: 2 Hypogammaglobulinemia: 1 |
Fever: 14 Diarrhea: 8 Abdominal pain: 7 |
C. coli (15) C. fetus (6) C. jejuni (3) |
Ampicillin-Sulbactam: (18/24) Cefotaxime: (0/24) Ciprofloxacin: (8/24) Levofloxacin: (9/24) Ertapenem: (12/24) Meropenem: (24/24) Erythromycin: (5/24) |
Cephalosporin: 13 Fluoroquinolone: 5 Amoxicillin/Clavulanate: 3 Macrolide: 2 |
Recovered: 21 Mortality: 3 |
| Abe et al.(2012) [10] | Japan | 1 | Pheochromocytoma: 1 | Fever, Fatigue | C. fetus | ND | Meropenem→Ciprofloxacin | Recovered |
| Shimizu et al.(2012) [11] | Japan | 2 | ESRD: 2 |
Fever: 2 Diarrhea: 2 |
C. upsaliensis (1) C. fetus (1) |
Ceftriaxone: (2/2) Gentamicin: (2/2) Erythromycin: (2/2) Levofloxacin: (0/2) |
Ceftriaxone+Gentamicin for C. upsaliensis Meropenem for C. fetus |
Recovered: 2 |
| Lang et al.(2009) [12] | Taiwan | 1 | ESRD: 1 | ND | C. jejuni | ND | Ciprofloxacin | Recovered |
| Okada et al.(2008) [13] | Japan | 1 | XLA: 1 | Fever, Leg pain | C. coli |
Cefotaxime: R Gentamicin: S Imipenem, Meropenem: S Erythromycin: I Ciprofloxacin: S |
Imipenem, Meropenem, Gentamicin, Ciprofloxacin, Minocycline, Azithromycin, Kanamycin | Recovered |
| Arai et al.(2007) [14] | Japan | 1 | XLA: 1 | Fever, Arthritis | C. coli |
Ceftazidime: S Erythromycin: R |
Ceftazidime→Minocycline Cefepime and Meropenem for relapse |
Recovered |
| Jirapongsananuruk et al.(2006) [15] | Thailand | 1 | XLA: 1 | Fever, Dyspnea | C. lari |
Aminoglycoside: S Cefepime: S 1–3rd cephalosporin: R Fluoroquinolone: R |
3rd cephalosporin+aminoglycoside, Amoxicillin/Clavulanate | Recovered |
| Teh et al.(2004) [16] | Singapore | 1 | Ovarian vein thrombosis: 1 | Fever, Loin pain | C. fetus |
Ampicillin: S Aminoglycoside: S |
Ceftriaxone→Clarithromycin | Recovered |
| Chuman et al.(2003) [17] | Japan | 1 | Adult T cell leukemia: 1 | Fever | C. fetus | ND | Cefoperazone/Sulbactam | Death after 1 month due to tumor progression |
| Woo et al.(2002) [18] | Hong Kong | 8 |
Liver cirrhosis: 4 Hepatocellular carcinoma: 2 Heart valve disease: 2 CKD: 1 |
Fever: 6 Diarrhea: 2 Abdominal pain: 3 |
C. fetus |
Ampicillin: (8/8) Gentamicin: (8/8) Ciprofloxacin: (7/8) Imipenem: (8/8) |
Penicillin+Gentamicin: 2 Ampicillin+Gentamicin: 1 Cefuroxime: 2 Amoxicillin/Clavulanate: 2 Ticarcillin/Clavulanate: 1 |
Recovered: 6 Mortality: 2 |
| Total |
Japan : 16 Hong Kong : 8 Taiwan : 25 Singapore : 1 Thailand : 1 |
51 |
Underlying disease present: 49 No underlying disease: 2 |
C. fetus (19) C. coli (17) C. jejuni (11) Others (4) |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download