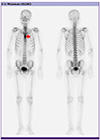
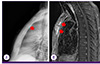
1. Platt MA, Ziegler K. Primary sternal osteomyelitis with bacteremia and distal seeding. J Emerg Med. 2012; 43:e93–e95.

2. Mofredj A, Guerin JM, Leibinger F, Masmoudi R. Primary sternal osteomyelitis and septicaemia due to Staphylococcus aureus. Scand J Infect Dis. 1999; 31:98–100.
3. Vacek TP, Rehman S, Yu S, Moza A, Assaly R. Another cause of chest pain: Staphylococcus aureus sternal osteomyelitis in an otherwise healthy adult. Int Med Case Rep J. 2014; 7:133–137.
4. Gill EA Jr, Stevens DL. Primary sternal osteomyelitis. West J Med. 1989; 151:199–203.
5. Lin JC, Miller SR, Gazzaniga AB. Primary sternal osteomyelitis. Ann Thorac Surg. 1996; 61:225–227.

6. Kara A, Tezer H, Devrim I, Caglar M, Cengiz AB, Gür D, Secmeer G. Primary sternal osteomyelitis in a healthy child due to community-acquired methicillin-resistant
Staphylococcus aureus and literature review. Scand J Infect Dis. 2007; 39:469–472.

7. Lo WK, Whimbey EE, Walsh GL. Primary sternal osteomyelitis presenting as a pleural-based mass. Chest. 1993; 103:1912–1913.

8. Kelly CA, Chetty MN. Primary sternal osteomyelitis. Thorax. 1985; 40:872–873.

9. Lee JH, Jeon SC, Jang HJ, Kim H, Kim YH, Chung WS. Primary sternal osteomyelitis caused by
Actinomyces israelii. Korean J Thorac Cardiovasc Surg. 2015; 48:86–89.

10. Chen YL, Tsai SH, Hsu KC, Chen CS, Hsu CW. Primary sternal osteomyelitis due to
Peptostreptococcus anaerobius. Infection. 2012; 40:195–197.

11. Baraboutis IG, Argyropoulou A, Papastamopoulos V, Psaroudaki Z, Paniara O, Skoutelis AT. Primary sternal osteomyelitis caused by
Nocardia nova: case report and literature review. Braz J Infect Dis. 2008; 12:257–259.

12. de Carli DM, Severo MD, Haygert CJ, Guollo M, Omairi A, Pedro VD, Silva EP, Rodrigues AT. Sternal osteomyelitis caused by infection with Mycobacterium tuberculosis
. J Bras Pneumol. 2009; 35:709–712.
13. Cherif E, Ben Hassine L, Boukhris I, Khalfallah N. Sternal tuberculosis in an immunocompetent adult. BMJ Case Rep. 2013; 2013:pii:bcr2013008810.

14. Yi IH, Youn HC, Kim DH, Kim SC, Kim BS, Cho KS, Park JC, Kwak YT. Primary sternal osteomyelitis: a case report. Korean J Thorac Cardiovasc Surg. 2006; 39:340–342.
15. Im HJ, Kim YK, Lee SM, Lee WW, Kim SE. Chronic osteomyelitis in sternum mimicking bone metastasis of lung cancer patient. Nucl Med Mol Imaging. 2009; 43:245–249.
16. Pineda C, Vargas A, Rodriguez AV. Imaging of osteomyelitis: current concepts. Infect Dis Clin North Am. 2006; 20:789–825.

17. Korean Society for Chemotherapy, Korean Society of Infectious Diseases, Korean Orthopaedic Association. Clinical guidelines for the antimicrobial treatment of bone and joint infections in Korea. Infect Chemother. 2014; 46:125–138.