This article has been
cited by other articles in ScienceCentral.
Abstract
Emphysematous osteomyelitis, especially that involving the extra-axial skeleton, is an extremely rare presentation but associated with significant morbidity and mortality. Here, we report a case in which a 58-year-old female patient with diabetes mellitus presented with emphysematous osteomyelitis that involved the sternum, clavicle, and pelvic bone and was caused by Escherichia coli via hematogenous spread of urinary tract infection. We successfully treated her with urgent and aggressive surgical drainage with prolonged antibiotics therapy. Early diagnosis and immediate surgical intervention are required for better outcomes in cases of emphysematous osteomyelitis.
Go to :

Keywords: Emphysematous osteomyelitis, Escherichia coli, Urinary tract infection
Introduction
Although intraosseous gas in the vertebral bodies or disc space is mostly associated with a non-infectious cause, intraosseous gas in the extra-axial skeleton in the absence of direct communication is virtually pathognomonic for emphysematous osteomyelitis [
1]. The first case of emphysematous osteomyelitis was reported in 1981 [
2], and only 29 cases have been reported globally thus far. To the best of our knowledge, no case of emphysematous osteomyelitis has been reported from Korea.
Here, we report the case of a 58-year-old female patient with poorly controlled diabetes mellitus, who presented with emphysematous osteomyelitis involving the sternum, clavicle, and pelvic bone due to Escherichia coli that had spread hematogenously from a urinary tract infection.
Go to :

Case Report
A 58-year-old woman was admitted to the emergency department owing to generalized weakness, chilling sensation and myalgia since the previous 10 days. She had a medical history of uncontrolled diabetes mellitus and hypertension. She took a prescription for diabetes mellitus, but did not take well. She had no history of trauma or surgery. Her vital signs were as follows: blood pressure, 90/40 mmHg; pulse rate, 100/min; respiratory rate, 20/min; and body temperature, 36.5°C. Laboratory data showed that a white blood cell count 24,290/mm
3 with 93% neutrophils, hemoglobin 11.7 g/dL, platelet count 57,000/mm
3 and C-reactive protein 33.2 mg/dL. Liver function enzymes showed aspartate aminotransferase 22 U/L, alanine aminotransferase 17 U/L, alkaline phosphatase 1,303 U/L, gamma glutamyl transferase 135 U/L, and total bilirubin 1.11 mg/dL. The HbA1c level was 11.9%, and serum glucose level was 665 mg/dL. Urinalysis showed many bacteria. Blood and urine cultures were obtained prior to initiation of empirical antibiotics. The chest radiograph showed a prominent heart size but no apparent lung parenchymal lesion. The chest and abdominal computed tomography (CT) scan revealed intraosseous gas in the sternum and left clavicle and intra-muscular gas in the left pectoralis major muscle as well as soft tissue emphysema involving the left shoulder and both chest walls (
Fig. 1A and 1B). Additionaly, intraosseous gases were noted in the right iliac bone and sacroiliac joint (
Fig. 1C). Right pyelonephritis with abscess at the right lower kidney was observed as well (
Fig. 1D).
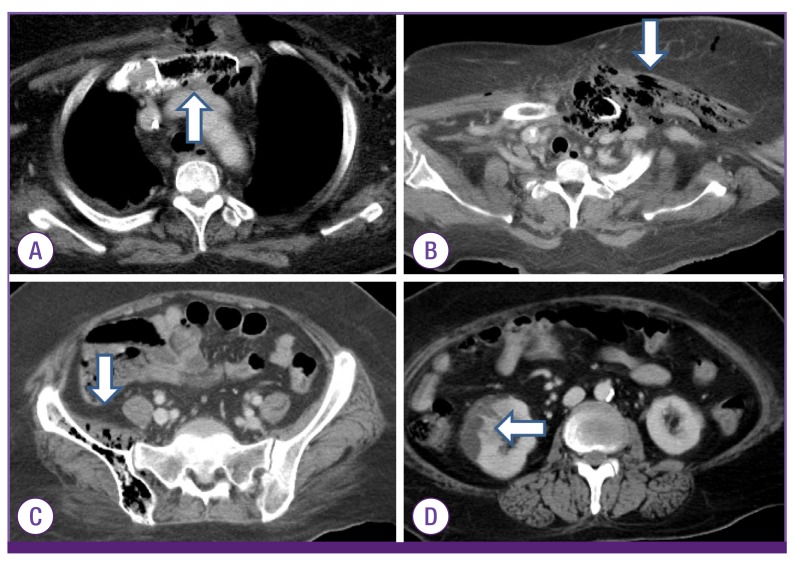 | Figure 1The chest and abdominal computed tomography (CT) scan at admission showed intraosseous gases involving the sternum (arrow) (A), left clavicle with swelling and intra-muscular gases in the adjacent muscle and soft tissues (arrow) (B). Intraosseous gases are noted in the right iliac bone and sacroiliac joint (arrow) (C). The CT scan showed a hypodense wedge-shaped lesion in the right lower kidney,(arrow) which indicated acute pyelonephritis with abscesses (D). 
|
Empirical antibiotic therapy with intravenous meropenem (1 g q8h, iv) and teicoplanin (800 mg q24h for 3 days and then 400 mg q24h, iv) was initiated and urgent surgical drainage was performed. At the time of surgical decompression, the pus from sternum, left clavicle and pelvic bone had a foul odor. The pus revealed extended-spectrum ß-lactamase non-producing E. coli, and both blood and urine culture grew E. coli with same antimicrobial susceptibility. Antibiotics were changed to piperacillin/tazobactam (4.0/0.5 g q8h, iv).
A follow-up CT conducted 4 weeks after admission showed marked regression of intraosseous gas in the sternum, left clavicle and adjacent extensive soft tissue emphysema in the left side of neck, left axilla, and both anterior chest walls. However, slightly increased size of abscess pocket involving the right iliacus muscle and osteomyelitis involving the right iliac bone were still noted. Therefore, catheter drainage of right iliac area was maintained for 8 weeks, and pus drainage at the right iliacus muscle was performed again and antibiotic therapy was continued. Follow-up CT scan after 8 weeks revealed emphysematous osteomyelitis of the sternum, left clavicle and right iliac bone with adjacent cellulitis almost resolved (
Fig. 2).
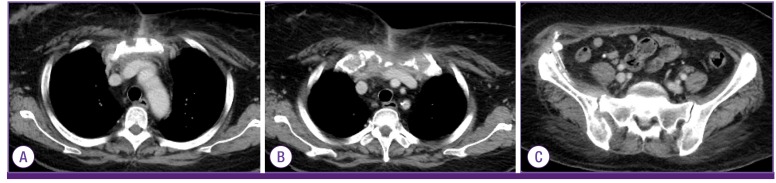 | Figure 2Follow-up chest and abdominal computed tomography scans at 12 weeks after admission showed emphysematous osteomyelitis in sternum and left clavicle with adjacent cellulitis are almost improved (A,B). Also, emphysematous osteomyelitis in the right iliac bone and sacroiliac joint are almost resolved (C). 
|
After 13 weeks of intravenous antibiotic therapy, the patient showed clinical improvement and was discharged.
Go to :

Discussion
Intraosseous gas in the extra-axial skeleton is rare and pathognomonic for emphysematous osteomyelitis. Although intravertebral gas is considered to derive from degenerative disease, or less commonly, osteonecrosis or neoplasm [
345], serious infection should be considered in cases of extensive intravertebral gas, bone edema or adjacent fluid collections.
In 1981, for the first time, Ram and colleagues described three patients with emphysematous osteomyelitis involving the tibia, pelvis, and fibula, respectively [
2]. Until now, only 29 cases have been reported in the English literature globally [
1678]. The involving sites of osteomyelitis of reported cases were vertebrae, pelvis, sacrum, femur, tibia, fibula, and midfoot [
1678]. To the best of our knowledge, emphysematous osteomyelitis has not previously been described in Korea.
In most cases, the infection occurs by hematogenous spread, however may also be related to contagious spread from an intraabdominal source of infection, or from a skin or soft tissue source of infection; or following intra-abdominal or spinal surgery [
7]. In this case, a urinary tract infection was the possible source of hematogenous spread that resulted in emphysematous osteomyelitis. Underlying comorbidities such as diabetes mellitus and malignancy are known to compromise immune function [
1]. In this case, the patient had poorly controlled diabetes mellitus, that was a risk factor for emphysematous osteomyelitis. Common causative organisms include members of the Enterobacteriaceae family or anaerobes (especially
Fusobacterium necrophorum). The monomicrobial causes of emphysematous osteomyelitis are similar to causes of other gas-forming infections, which include
E. coli, Klebsiella pneumoniae, Enterobacter aerogenes, and
Clostridium spp
. [
191011]. In cases of post-surgical infections, gram-positive organisms such as
Staphylococcus aureus, non-hemolytic streptococci, and enterococci, and
Pseudomonas spp. may be the causative organisms [
1]. Of all the reported cases emphysematous osteomyelitis, the causative organism was
E. coli in 8 cases (4 cases of monomicrobial infection and 4 cases of polymicrobial infection due to
E. coli and other organisms) [
1678].
In cases of acute osteomyelitis, appropriate antimicrobial agents should be given promptly in cases of bacteremia, bone necrosis and bone destruction [
12]. And surgical treatment should be considered in acute osteomyelitis if there is finding of abscess formation or radiologic evidence of necrosis, or if the patients do not respond to antimicrobial agents [
12]. The duration of treatment must be tailored according to the condition of the individual patient, however duration of at least 4–6 weeks of antimicrobial treatment after the last debridement is recommended [
12].
Emphysematous osteomyelitis is associated with high morbidity and mortality, as high as 32%, especially in patients with diabetes mellitus [
1]. A diagnosis of emphysematous osteomyelitis with the presence of intraosseous gas in the extra-axial skeleton suggests a severe infection that requires aggressive and immediate treatment [
13].
In conclusion, we report a rare case of emphysematous osteomyelitis of the sternum, clavicle and pelvic bone due to E. coli that had spread hematogenously from a urinary tract infection. Early diagnosis and urgent and aggressive surgical intevention as well as broad antibiotics are crucial for survival of patients.
Go to :







 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download