Abstract
Human metapneumovirus is known to be similar to respiratory syncytial virus. Because of an incomplete protective immune response to new genotypes, re-infection occurs frequently, especially in the elderly. However, the clinical manifestations of human metapneumovirus need to be further characterized in adults. A 73-year-old woman presented to the emergency room with acute dyspnea, chest discomfort and influenza-like illness. The patient was diagnosed with human metapneumovirus infection, complicated by pneumonia and myopericarditis. With supportive care including oxygen supplementation, the patient recovered completely without any serious sequelae. Human metapneumovirus infection may contribute to the development of cardiovascular manifestations, particularly in the elderly population.
Human metapneumovirus (hMPV), a paramyxovirus similar to respiratory syncytial virus (RSV), was first described in 2001 [1]. Although hMPV is primarily known as a causative agent of respiratory tract infections in children, the virus can also cause respiratory infections in adults [2]. Because of an incomplete protective immune response to new genotypes, reinfection occurs frequently, especially in the elderly [2]. However, the clinical manifestations of hMPV need to be further characterized in adults. Here, we report a case of hMPV infection in an elderly patient that was complicated by acute myopericarditis.
A 73-year-old woman presented to the emergency room with fever, cough, chest discomfort and progressive dyspnea in early April. The patient had suffered from an influenza-like illness for 4 days before presentation. She had underlying diabetes mellitus and hypertension, and was diagnosed with atrial fibrillation 7 days prior to admission.
On physical examination the patient was slightly dyspneic with a heart rate of 72 beats/min, respiratory rate of 24 breaths/min, blood pressure of 160/77 mmHg and tympanic body temperature of 38.4℃. Lung auscultation revealed crackle in both lower lung fields. Initial laboratory tests showed mild leukopenia, thrombocytopenia, and anemia (Table 1). Serum C-reactive protein (97.57 mg/L) and erythrocyte sedimentation rate (52 mm/h) were elevated, but liver function tests were within normal limits. Hypoxia (oxygen saturation, 88% in room air) was observed on arterial blood gas analysis. Levels of creatine kinase-MB (CK-MB, 7.9 ng/mL) and Pro-B type natriurectic peptide (pro-BNP, 2618 pg/mL) were elevated. Chest X-ray showed cardiomegaly and diffuse opacity in both lower lungs (Fig. 1A). Electrocardiogram showed atrial fibrillation with a normal ventricular response, and echocardiography showed slightly decreased systolic function (ejection fraction, 50-55%) with a small amount of pericardial effusion that was not present 7 days previously at the time of diagnosis of atrial fibrillation. Chest computed tomography (CT) showed ground-glass opacities in bilateral lungs, right-sided pleural effusion, and pericardial effusion (Fig. 2). Ultrasound-guided aspiration of pleural effusion revealed a lymphocyte-dominant exudate with pH 7.0, WBC 560 cells/L with relative lymphocytosis (95%), glucose 104 mg/dL, protein 2.8 g/dL, lactate dehydrogenase (LDH) 356 IU/L and adenosine deaminase (ADA) 18.3 IU/L. Pericardiocentesis could not be performed because of safety concerns.
Initially, single-dose intravenous peramivir (300 mg) was given to the patient assuming influenza viral pneumonia. Because of concerns about concomitant bacterial pneumonia, antibiotics were also administered (ceftriaxone 2.0 IV every 24 hours for 7 days and azithromycin 250mg orally bid for 3 days).
A throat swab sample was taken from the patient and polymerase chain reaction (PCR) was performed to detect respiratory viruses using Seeplex® RV15 ACE Detection kit (Seegene, Seoul, Korea). PCR analysis was positive only for hMPV on the fourth day after hospitalization; tests for other respiratory viruses (influenza virus, RSV, parainfluenza virus, coronavirus, rhinovirus, enterovirus, adenovirus, and bocavirus) were negative. Laboratory tests [sputum gram stain and culture, blood cultures, urinary antigen tests for Streptococcus pneumoniae, and serologic tests for atypical pathogens (Chlamydophila pneumoniae, Mycoplasma pneumoniae, and Legionella species)] were performed on suspicion of concomitant bacterial pneumonia on admission, but none gave positive results.
Finally, the patient was diagnosed with hMPV infection complicated by pneumonia and myopericarditis. With supportive care including oxygen supplementation, the fever subsided after 2 days of hospitalization and shortness of breath improved progressively. Pneumonia and cardiomegaly were improved on chest X-ray (Fig. 1B). Systolic function was normalized (ejection fraction, 60-65%), and pericardial effusion disappeared on follow-up echocardiography (7th day after hospitalization). The patient recovered without any serious sequelae.
hMPV is a newly discovered respiratory pathogen belonging to the Metapneumovirus genus within the Paramyxoviridae family [2]. hMPV is distributed worldwide and shows seasonal variation, peaking in the late winter and early spring [3]. Particularly in Korea, as reported during early April in this case, hPMV co-circulate with influenza B in spring from late March to early May [4]. hMPV is most prevalent in the pediatric population with almost 100% seroprevalence at the age of five, but with advances in diagnostic technology the virus is increasingly becoming recognized as a significant pathogen in older adults [2]. We experienced an interesting case of hMPV infection complicated by acute myopericarditis that was resolved after conservative management.
Although hMPV is known to cause various upper and lower respiratory syndromes including common cold, bronchiolitis, pneumonia, and asthma exacerbation, the full spectrum of clinical manifestations remains to be clarified [2]. Similar to influenza A virus, hMPV infection can cause primary viral pneumonia, and it be accompanied by concomitant bacterial pneumonia also [456]. In a mouse model, hMPV infection facilitated severe bacterial infection by obstruction of higher levels of airways and induction of inflammatory cytokines [7]. Compared with influenza A infection, the impairment of pneumococcal clearance in the mouse model was shorter for hMPV infection [8]. In the present study, although a bacterial pathogen was not isolated, antibiotics were administered because of concerns regarding concomitant bacterial pneumonia.
Although respiratory tract infections are the most common manifestations of hMPV infection, cardiovascular problems may be a feature that distinguishes hMPV infection from other respiratory viral infections; cardiovascular diseases were more common in patients with hMPV infections compared to those with influenza and RSV infections [910]. Previously, a Korean pediatric study identified hMPV as one of the etiologic agents of acute myocarditis [11], while a Japanese group suggested an association between hMPV seroprevalence and hypertension in elderly subjects [12]. It is not clear whether the virus has a tropism for the myocardium or patients with underlying cardiovascular disease are more vulnerable to hMPV. In either case, myocardial involvement can represent a life-threatening condition and the association between hMPV and cardiovascular diseases needs to be further clarified with respect to both host and virus.
This report has several limitations. First, myocardial and pericardial biopsy was not taken because of safety concerns; therefore, pathologic confirmation of the diagnosis of myopericarditis was not possible. Second, CK-MB was elevated, but cardiac troponin levels were not checked. The elevations of cardiac troponin I or T levels are known to be more common than CK-MB elevation in patients with biopsy-proven myocarditis, and reflect acute, early-onset myocarditis [1314]. Third, cardiac magnetic resonance (CMR) imaging was not taken in this case. CMR imaging has emerged as the most important diagnostic tool of myocarditis, distinguishing between ischemic and non-ischemic cardiomyopathy [1516]. Although CMR image was not available however, coronary angiography showed normal appearance of coronary arteries only with mild atherosclerosis. Finally, bronchoscopy was not taken, so respiratory virus PCR was just carried out with upper respiratory specimen. Although clinically compatible with hMPV pneumonia, positive PCR with throat swab specimen would not mean lower respiratory tract infection.
In conclusion, hMPV infection may contribute to the development of cardiovascular manifestations, particularly in the elderly population. We should consider the possibility of myocardial involvement in adult patients with hMPV infection if they have suspicious symptoms or signs.
Figures and Tables
Figure 1
Chest X-ray findings show cardiomegaly and diffuse opacity in both lower lungs with the dominance on right lower lobe (A), and marked improvement after a 7-day conservative treatment (B).
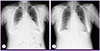
Figure 2
Chest computed tomography shows ground-glass opacity in both lower lungs (A) and pericardial effusion with right-sided pleural effusion (B).
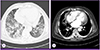
Table 1
Laboratory findings at initial presentation

WBC, white blood cells; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; CPK, creatine phosphokinase; LDH, lactate dehydrogenase; BUN, blood urea nitrogen; ABGA, arterial blood gas analysis; CK-MB, creatine kinase-MB; BNP, B-type natriuretic peptide.
References
1. van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RA, Osterhaus AD. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001; 7:719–724.

2. Haas LE, Thijsen SF, van Elden L, Heemstra KA. Human metapneumovirus in adults. Viruses. 2013; 5:87–110.

3. Kahn JS. Human metapneumovirus: a newly emerging respiratory pathogen. Curr Opin Infect Dis. 2003; 16:255–258.

4. Seo YB, Song JY, Choi MJ, Kim IS, Yang TU, Hong KW, Cheong HJ, Kim WJ. Etiology and clinical outcomes of acute respiratory virus infection in hospitalized adults. Infect Chemother. 2014; 46:67–76.

5. Yoo JY, Eun JY, Lee EJ, Kim TH, Choo EJ, Jeon MH. A case of human metapneumovirus pneumonia in an immunocompetent adult patient mimicking with influenza (A/H1N1-2009) pandemic. Infect Chemother. 2011; 43:217–221.

6. Lim HJ, Lee JW, Park YS, Kim NH, Kim M, Yim JJ, Yang SC, Yoo CG, Kim YW, Han SK, Shim YS, Lee SM. A case of severe human metapneumovirus pneumonia requiring mechanical ventilation in an immunocompetent adult. Tuberc Respir Dis. 2009; 67:135–139.

7. Kukavica-Ibrulj I, Hamelin ME, Prince GA, Gagnon C, Bergeron Y, Bergeron MG, Boivin G. Infection with human metapneumovirus predisposes mice to severe pneumococcal pneumonia. J Virol. 2009; 83:1341–1349.

8. Ludewick HP, Aerts L, Hamelin ME, Boivin G. Long-term impairment of Streptococcus pneumoniae lung clearance is observed after initial infection with influenza A virus but not human metapneumovirus in mice. J Gen Virol. 2011; 92:1662–1665.

9. Johnstone J, Majumdar SR, Fox JD, Marrie TJ. Viral infection in adults hospitalized with community-acquired pneumonia: prevalence, pathogens, and presentation. Chest. 2008; 134:1141–1148.

10. Widmer K, Zhu Y, Williams JV, Griffin MR, Edwards KM, Talbot HK. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J Infect Dis. 2012; 206:56–62.

11. Kim HJ, Yoo GH, Kil HR. Clinical outcome of acute myocarditis in children according to treatment modalities. Korean J Pediatr. 2010; 53:745–752.

12. Zeng L, Chen R, Ishigami K, Atsumi M, Koizumi Y, Sato K, Iritani O, Okuro M, Morimoto S. Association between human metapneumovirus seroprevalence and hypertension in elderly subjects in a long-term care facility. Hypertens Res. 2011; 34:474–478.

13. Lauer B, Niederau C, Kühl U, Schannwell M, Pauschinger M, Strauer BE, Schultheiss HP. Cardiac troponin T in patients with clinically suspected myocarditis. J Am Coll Cardiol. 1997; 30:1354–1359.

14. Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Experimental and clinical correlates. Circulation. 1997; 95:163–168.

15. Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013; 6:833–839.

16. Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P. International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009; 53:1475–1487.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download