This article has been
cited by other articles in ScienceCentral.
Abstract
Background
The World Health Organization recommends the surveillance of influenza-like illness (ILI) and severe acute respiratory infection (SARI) to respond effectively to both seasonal influenza epidemics and pandemics. In Korea, the “Hospital-based Influenza Morbidity and Mortality (HIMM)” surveillance system has been operated to monitor ILI and SARI occurrences.
Materials and Methods
A multi-center prospective observational study was conducted. Adult patients with acute respiratory infection (ARI) were enrolled during the 2011-12, 2012-2013, and 2013-2014 influenza seasons at the 10 university hospitals using the HIMM surveillance system. With respect to SARI and pneumonia development, risk profiles were analyzed in patients with ARI in Korea.
Results
A total of 5,459 cases were eligible for this analysis. Among 5,459 cases with ARI, 2,887 cases (52.9%) were identified that they had influenza infection. Among enrolled cases, 750 cases belonged to the SARI group, while 4,709 cases belonged to the non-SARI group. With respect to pneumonia development, 317 cases were accompanied by pneumonia, and 5,142 cases were not. Multivariate analyses revealed that the following factors were associated with an increased risk of SARI: Old age (≥65 years) (odds ratio [OR] 2.69, 95% confidence interval [CI] 2.2-3.32), chronic heart disease (CHD) (OR 2.24, 95% CI 1.68-2.98), cerebrovascular disease (CVD) (OR 1.49, 95% CI 1.05-2.10), chronic obstructive pulmonary disease (COPD) (OR 2.34, 95% CI 1.48-3.69), asthma (OR 2.33, 95% CI 1.62-3.36), chronic kidney disease (CKD) (OR 2.62, 95% CI 1.73-3.99), chronic liver disease (OR 1.71, 95% CI 1.04-2.81), and autoimmune diseases (OR 2.53, 1.57-4.08). Multivariate analyses revealed that the following factors were independent risk factors for pneumonia development: Old age (≥65 years) (OR 5.71, 95% CI 4.10-7.94), CHD (OR 1.54, 95% CI 1.07-2.22), COPD (OR 2.34, 95% CI 1.48-3.69), asthma (OR 2.33, 95% CI 1.62-3.36), CKD (OR 2.62, 95% CI 1.73-3.99), immunocompromised conditions (OR 3.12, 95% CI 1.47-6.62), and autoimmune diseases (OR 3.35, 95% CI 1.79-6.27). The risk of SARI and pneumonia was increased by the number of concurrent chronic medical conditions.
Conclusion
The risk of SARI and pneumonia development among adult patient with ARI was significantly increased by the presence or number of concurrent chronic medical conditions in Korea.
Keywords: Influenza, Complication, Hospitalization, Pneumonia, Risk factors
Introduction
Respiratory illness due to the influenza virus and other respiratory pathogens is a major cause of morbidity and mortality worldwide. According to the World Health Organization (WHO), these annual epidemics result in 3–5 million cases of severe illness and 250–500 thousand deaths worldwide [
1]. In particular, the proportion of acute respiratory illness (ARI) cases due to influenza becomes higher in the winter season with increased morbidity and mortality [
23].
Patients with influenza are known to present with influenza-like illness (ILI), which includes fever and respiratory symptoms. However, some patients show atypical manifestations. Although many hospitalizations are due to pneumonia (viral and bacterial), influenza is also complicated by acute exacerbation of cardiovascular and pulmonary diseases. Moreover, neuromuscular complications, such as encephalopathy, transverse myelitis, and aseptic meningitis, have been reported in influenza patients [
45].
Influenza surveillance systems are essential components of public health systems. WHO recommends the surveillance of ILI and severe acute respiratory infection (SARI) to respond effectively to both seasonal influenza epidemics and pandemics [
6]. Some studies, however, have reported that laboratory-confirmed influenza (LCI) with complications is not completely compatible with ILI criteria [
478]. Although most of the current surveillance systems are based on ILI, some patients do not present with typical ILI, especially elderly patients [
9]. In this aspect, the usual criteria of ILI and SARI are limited in their ability to predict the risk of influenza-related complications or hospitalization.
In South Korea, the Transgovernmental Enterprise for Pandemic Influenza in Korea (TEPIK) has existed since 2011. TEPIK developed the “Hospital-based Influenza Morbidity and Mortality” (HIMM) surveillance system, which consists of an integrated clinical and laboratory surveillance system. Currently, 10 university hospitals use this system on a nationwide scale. This system monitors not only influenza activity but also the severity of influenza, such as hospitalizations, complications, and mortality. It is important to monitor the severity of influenza and collect viral isolates in order to analyze the virulence of circulating strains. Additionally, this system can detect and track the course of influenza cases with typical ILI as well as non-ILI.
In this study, therefore, we aimed to identify risk factors for SARI and pneumonia among adult patients with ARI during the 2011-2014 influenza seasons using HIMM surveillance data in Korea.
Materials and Methods
1. Study design
A prospective observational design was used. The study period extended through the 2011-2012, 2012-2013, and 2013-2014 influenza seasons. Influenza season was defined from October 1 to the first week of May in accordance with the recommendations of Centers for Disease Control and Prevention (CDC) [
10]. Analyses were performed according to the week of the year, with Week 1 defined as the week (from Monday through Sunday) in which January 1 fell. The study conducted at the 10 tertiary university hospitals, including Guro Hospital of Korea University College of Medicine, Ansan Hospital of Korea University College of Medicine, St. Vincent's Hospital of College of Medicine, the Catholic University of Korea, Hospital of Kyungpook National University School of Medicine, Hospital of Pusan National University School of Medicine, Hospital of Chonnam National University School of Medicine, Hospital of Chungbuk National University School of Medicine, and Kangnam Sacred Heart Hospital, Hallym University School of Medicine. Participating sites prospectively enrolled the data of adult patients (>18 years of age) who visited the emergency room with an ARI.
2. Definitions
ARI was defined as follows: (1) one or more respiratory symptom (cough, sore throat, shortness of breath) (2) considered by the doctor to be due to infection. ILI, according to the Centers for Disease Control and Prevention (CDC), was defined as a history of fever or sudden onset of fever (>37.8°C, within 7 days) and a cough and/or sore throat without a known acute respiratory infection other than influenza. The following SARI case definition was used: (1) history of fever or sudden onset of fever (>37.8°C, within 7 days), (2) one or more respiratory symptom (cough, sore throat, rhinorrhea), (3) shortness of breath or difficulty breathing, and (4) admission [
11]. However, the HIMM system uses “modified SARI,” which is defined as either (A) classical SARI by WHO or (B) non-ILI SARI with laboratory-confirmed influenza-related admission. A rapid antigen test (SD Bioline Rapid Influenza Test, Standard Diagnostics, Inc., Yongin, Korea) and a 15 respiratory virus (RV) polymerase chain reaction (PCR) test (Seeplex
® RV15 ACE Detection, Seegene INc., Seoul, Korea) using nasopharyngeal swabs were performed for the diagnosis of LCI. This study was approved by the Korea University Guro Hospital Institutional Review Board [KUGH11088-001].
3. Study measures
The following information was recorded: age, sex, episodes of pneumonia and other complications, medical history, etc. In addition, we classified the admitted patients into seven different groups based on their reason for admission regardless of whether they were diagnosed as classical SARI or modified SARI.
Group A: Lower respiratory infection at image (pneumonia)
B: Other respiratory infection complication (acute epiglottitis, acute otitis media, laryngitis, vestibular neuritis, bronchiolitis)
C: Encephalopathy, transient ischemic attack (TIA), spontaneous subdural hematoma (SDH)
D: Acute myocardial infarction (AMI), myocarditis, pericardial effusion
E: Severe dehydration (poor oral intake plus skin turgor plus acute kidney injury)
F: Aggravation of chronic disease (asthma, chronic obstructive pulmonary disease (COPD), chronic liver disease, chronic kidney disease (CKD), cardiovascular disease)
G: Other severe subjective symptom (myalgia, poor oral intake, threatened abortion)
4. Statistical analysis
Risk factors for SARI and pneumonia were analyzed. Continuous variables were compared by the Student’s independent t-test, while categorical variables were compared using the chi-square test. Multivariate analyses were performed using stepwise logistic regression analysis for risk factors of pneumonia and SARI, and the risk ratios with a 95% confidence interval (CI) were calculated. Factors found to be significant in the univariate logistic regression analysis were included as independent variables in the multivariate logistic regression. In addition, we calculated the odd ratios (ORs) for SARI and pneumonia between the cases who had multiple risk factors and those who did not have any risk factors. In this analysis, the risk factors were counted equally regardless of type. All analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
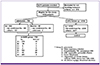
A total of 6,275 cases were registered to the study in the 2011-2014 influenza seasons. Eight patients who did not have ARI and 816 patients who had ARI but had unknown past medical histories were excluded (
Fig. 1). As a result, 5,459 cases were eligible for this analysis. Among 5,459 cases, 2,887 cases (52.9%) were identified that they had LCI. Other viruses were summarized in
Supplementary Table 1.
Figure 1
Study flow
ILI, influenza-like illness; m-SARI, modified severe acute respiratory infection; flu, influenza; OPD, out-patient department; TIA, transient ischemic attack; SDH, subdural hematoma; AMI, acute myocardial infarction; COPD, chronic obstructive pulmonary disease; CKD, chronic kidney disease; CHD, chronic heart disease.
1. Characteristics of population
The baseline characteristics of enrolled cases are summarized in
Table 1. The proportion of females was higher than that of males (42.9%). The mean age of patients with ARI was 48.8 years. Although most patients with ARI were under 65 (72.8%), more than half (52%) had comorbidities. Among the 5,459 enrolled cases, 750 cases (13.7%) belonged to the modified SARI group, while 4,709 cases (86.3%) belonged to the non-SARI group (control). Among the 750 patients with modified SARI, 682 (91%) patients had ILI, and 68 patients did not meet the ILI definition (
Fig. 1). Specifically, in Group A, pneumonia was most common (317, 42.3%), followed by Group E (severe dehydration, 122, 16.3%), Group F (aggravation of chronic disease, 123, 16.4%), Group B (other respiratory infection complication, 31, 4.1%), Group C (brain complication, 11, 1.5%), and Group D (cardiovascular complications, 8, 1.1%).
Table 1
Baseline characteristics of enrolled adult cases with acute respiratory illness

|
Baseline demographic and clinical characteristics (total no. = 5,459) |
Data (n, %) |
|
Male sex - no. (%) |
2356 (42.9%) |
|
Age (year) - mean (range) |
48.8 (18-99) |
|
Age (group) - no. (%) |
3976 (72.8) |
|
18-64 years |
1483 (27.2) |
|
65-99 years |
|
|
Comorbidity - no. (%) |
2841 (52) |
|
Hypertension |
1197 (22.9) |
|
Diabetes mellitus |
670 (12.3) |
|
Solid cancer |
356 (6.5) |
|
Chronic heart disease |
322 (5.9) |
|
Cerebrovascular disease |
256 (4.7) |
|
Asthma |
163 (3) |
|
Chronic kidney disease |
156 (2.9) |
|
Chronic liver disease |
130 (2.4) |
|
Autoimmune disease |
113 (2.1) |
|
Chronic obstructive pulmonary disease |
|
|
Neuromuscular disease |
80 (1.5) |
|
Hematologic malignancy |
39 (<0.1) |
|
Solid organ transplantation |
11 (<0.1) |
|
Bone marrow transplantation |
1 (<0.1) |
|
HIV/AIDS |
17 (<0.1) |
2. Risk factors of modified SARI and pneumonia
When we analyzed the risk factors for modified SARI, the univariate analysis revealed the following factors were statistically significant: influenza vaccine, pneumococcal vaccine, old age (over 65 years), diabetes mellitus (DM), chronic heart diseases (CHD), cerebrovascular disease (CVD), neuromuscular disease, COPD, asthma, CKD, chronic liver disease, solid cancer, immunocompromised diseases, and autoimmune diseases (
Table 2). The multivariate analyses revealed the following factors were associated with an increased risk for modified SARI: old age (over 65 years) (OR 2.69, 95% CI 2.2-3.32), CHD (OR 2.24, 95% CI 1.68-2.98), CVD (OR 1.49, 95% CI 1.05-2.10), COPD (OR 2.34, 95% CI 1.48-3.69), asthma (OR 2.33, 95% CI 1.62-3.36), CKD (OR 2.62, 95% CI 1.73-3.99), chronic liver disease (CLD) (OR 1.71, 95% CI 1.04-2.81), and autoimmune diseases (OR 2.53, 1.57-4.08).
Table 2
Analysis of risk factors for severe acute respiratory infection among adult cases with acute respiratory illness

|
Control group (n = 4709) |
Modified SARI (n = 750) |
Univariate analysis |
Multivariate analysis |
|
P value |
Odds ratio (95% CI) |
|
Age, mean years (SD) |
46.7 (SD 19.2) |
61.6 (SD 18.9) |
<0.001 |
|
|
Male sex - no. (%) |
2,015 (42.8%) |
326 (43.5%) |
0.728 |
|
|
Influenza vaccinea
|
1,365 |
305 |
<0.001 |
0.84 (0.68-1.03) |
|
Pneumococcal vaccineb
|
564 |
92 |
<0.001 |
|
|
Age < 65 |
3291 |
270 |
<0.001 |
|
|
Age ≥ 65 |
743 |
164 |
<0.001 |
|
|
DM |
506 (10.7%) |
164 (3.5%) |
<0.001 |
0.94 (0.73–1.20) |
|
Chronic heart disease |
189 (4%) |
103 (13.7%) |
<0.001 |
2.24 (1.68–2.98) |
|
CVD |
17,177 (3.8%) |
79 (10.5%) |
<0.001 |
1.49 (1.05–2.10) |
|
Neuromuscular |
56 (1.2%) |
24 (3.2%) |
<0.001 |
1.2 (0.67–2.13) |
|
COPD |
48 (1%) |
45 (6%) |
<0.001 |
2.34 (1.48–3.69) |
|
Asthma |
94 (2%) |
69 (9.2%) |
<0.001 |
2.33 (1.62–3.36) |
|
CKD |
95 (2%) |
61 (8.1%) |
<0.001 |
2.62 (1.73–3.98) |
|
CLD |
101 (2.1%) |
29 (3.9%) |
<0.004 |
1.71 (1.04–2.81) |
|
Solid cancer |
276 (5.9%) |
80 (10.7%) |
<0.001 |
1.17 (0.86–1.59) |
|
Immunocompromised |
51 (1.1%) |
17 (2.3%) |
0.007 |
1.71 (0.92–3.20) |
|
-Hematologic malignancy |
30 |
9 |
|
-Solid organ transplant |
8 |
3 |
|
-BMT |
1 |
0 |
|
-HIV/AIDS |
13 |
4 |
|
Autoimmune disease |
79 (1.7%) |
34 (4.5%) |
<0.001 |
2.53 (1.57–4.09) |
With respect to pneumonia development, 317 cases were accompanied by pneumonia, and 5,142 cases were not. When we analyzed the risk factors for pneumonia, the univariate analysis revealed the following factors were statistically significant: influenza vaccine, pneumococcal vaccine, old age (over 65 years), DM, CHD, CVD, neuromuscular disease, COPD, asthma, CKD, CLD, solid cancer, immunocompromised diseases, and autoimmune diseases (
Table 3). The multivariate analyses revealed the following factors were independent risk factors for pneumonia development: influenza vaccine (OR 0.74, 95% CI 0.54-1.0), old age (≥65 years) (OR 5.71, 95% CI 4.10-7.94), CHD (OR 1.54, 95% CI 1.07-2.22), COPD (OR 2.34, 95% CI 1.48-3.69), asthma (OR 2.33, 95% CI 1.62-3.36), CKD (OR 2.62, 95% CI 1.73-3.99), immunocompromised conditions (OR 3.12, 95% CI 1.47-6.62), and autoimmune diseases (OR 3.35, 95% CI 1.79-6.27) (
Table 3).
Table 3
Analysis of risk factors for pneumonia development among adult cases with acute respiratory illness

|
Non-pneumonia (n = 5,142) |
Pneumonia (n = 317) |
Univariate analysis |
Multivariate analysis |
|
P value |
Odds ratio (95% CI) |
|
Age (year) - mean |
47.5 (SD 19.4) |
68.5 (SD 15.8) |
<0.001 |
|
|
Male sex - no. (%) |
2168 (42.2%) |
173 (54.6%) |
<0.001 |
|
|
Influenza vaccine a
|
1530 |
140 |
<0.001 |
0.74 (0.54-1.0) |
|
Pneumococcal vaccine b
|
274 |
52 |
<0.001 |
1.29 (0.87-1.92) |
|
Age < 65 |
3496 |
65 |
<0.001 |
|
|
Age ≥ 65 |
820 |
87 |
<0.001 |
|
|
DM |
577 (11.2%) |
93 (29.3%) |
<0.001 |
1.15 (0.84-1.59) |
|
Chronic heart disease |
255 (6.1%) |
67 (21.1%) |
<0.001 |
1.54 (1.07-2.22) |
|
CVD |
211 (4.1%) |
45 (14.2%) |
<0.001 |
1.37 (0.89-2.12) |
|
Neuromuscular |
63 (1.2%) |
17 (5.4%) |
<0.001 |
1.81 (0.94-3.5) |
|
COPD |
67 (1.3%) |
26 (8.2%) |
<0.001 |
2.01 (1.18-3.44) |
|
Asthma |
130 (2.5%) |
33 (10.4%) |
<0.001 |
2.13 (1.35-3.35) |
|
CKD |
116 (2.3%) |
40 (12.6%) |
<0.001 |
3.81 (2.36-6.16) |
|
CLD |
115 (2.3%) |
15 (4.7%) |
0.006 |
1.86 (0.93-3.5) |
|
Solid cancer |
319 (6.2%) |
37 (11.7%) |
<0.001 |
1.10 (0.71-1.71) |
|
Immunocompromised |
56 (1.1%) |
12 (3.8%) |
<0.001 |
3.12 (1.47-6.62) |
|
-Hematologic malignancy |
33 |
6 |
|
-Solid organ transplant, |
9 |
2 |
|
-BMT |
1 |
0 |
|
-HIV/AIDS |
14 |
4 |
|
Autoimmune disease |
95 (1.9%) |
18 (5.7%) |
<.001 |
3.35 (1.79-6.27) |
Furthermore, the risk of modified SARI and pneumonia was significantly increased by the number of concurrent chronic medical conditions. The risk ratio of modified SARI increased about 9.5 times as the number of SARI risk factors increased from zero to four. Moreover, the risk ratio of pneumonia increased about 16 times as the number of pneumonia risk factors increased from zero to four (
Fig. 2).
Figure 2
Risk of SARI and pneumonia following acute respiratory illness in adult cases with multiple risk factors
(A) The risk ratio of modified SARI increases about 9.5 times as the number of SARI risk factors increases from zero to four. (B) The risk ratio of pneumonia increases about 16 times as the number of pneumonia risk factors increases from zero to four.
SARI, severe acute respiratory infection.

Discussion
In this study, we found several independent risk factors related to hospitalization among adult patients with ARI at medical emergency departments (EDs) in Korea. In addition, we identified several independent risk factors for pneumonia development after ARI among adult patients who visited EDs in influenza season. The present study showed that old age and CKD were most strongly associated with modified SARI, while old age, immunocompromised conditions, and autoimmune diseases were strongly related to pneumonia. This study would be valuable in several aspects. First of all, this multi-centered study was conducted prospectively. HIMM has been operated for more than three influenza seasons; the data was collected by well-trained research nurses, and confirmed by investigational physicians in each hospital. Secondly, because non-ILI SARI cases were included, this study might be useful to understand the spectrum of severe influenza comprehensively.
Going one step forward, the risk of modified SARI and pneumonia was significantly increased by the number of concurrent chronic medical conditions (
Fig. 2). The Government of South Korea, however, has a national influenza immunization and pneumococcal vaccination program that just targets the elderly (aged over 65 years). Thus, we need to evaluate the cost-effectiveness of influenza and pneumococcal vaccination for young adults with multiple comorbidities.
Respiratory complications after influenza infection are known to be more common than non-respiratory complications [
5]. However in etiological studies of encephalitis, Influenza A and/or B have been identified in up to 8.5% of adult patients [
12] and in up to 10% of pediatric cases [
13]. In this study, 11 patients (1.7%) were identified as having LCI with encephalopathy, TIA, or spontaneous SDH among the total of 649 LCI patients. The proportion of patients manifesting central nervous system (CNS) complications is about 1.1% among ARI cases in flu season. In the case of cardiovascular complications, Guan et al. [
14] reported a 7.5 OR (95% CI 1.3-43.0) in Influenza A and a 27.3 OR (95% CI 6.6-113.8) in Influenza B. Cardiac complications, such as myocarditis, are well recognized, but the role of influenza as a trigger of acute myocardial infarction is less clear. In this study, we found that 11 (1.7%) patients were identified as having LCI with cardiac complications (AMI, myocarditis, or pericardial effusion) among the total of 649 LCI patients. In addition, among 12 patients who were classified as having aggravation of heart failure without myocarditis or myocardial infarction, 11 patients had influenza infections identified by laboratory tests. Because we classified aggravation of heart failure (11 patients, 1.7%) as Group F (aggravation of chronic diseases), if taken together, the impact of influenza on the cardiovascular system might be much greater. During influenza season, influenza should be considered a triggering factor for cardiovascular complications, cerebrovascular events, and other CNS complications.
This study has several limitations to be considered. First, a large proportion of patients had severe underlying diseases, because these centers are tertiary hospitals. Second limitation is that hospitalization is a complex outcome resulting from multiple components: the physician’s decision as well as the patient’s decision to seek medical attention, occupation, and economic problems. Depending on the circumstances, some of the extraneous factors may play an important role in the outcome (hospitalization) and thus make the interpretation of our results challenging.
In conclusion, old age and chronic medical conditions were independent risk factors for SARI and pneumonia, and the risk was significantly increased by the number of underlying comorbidities in Korea.
Acknowledgements
This work was supported by a grant from the Transgovernmental Enterprise for Pandemic Influenza in Korea, which is part of the Korea Healthcare Technology R&D Project being conducted by the Ministry of Health & Welfare, Korea [grant number A103001].
References
2. Elliot AJ, Cross KW, Fleming DM. Acute respiratory infections and winter pressures on hospital admissions in England and Wales 1990-2005. J Public Health (Oxf). 2008; 30:91–98.

3. Nielsen J, Mazick A, Glismann S, Mølbak K. Excess mortality related to seasonal influenza and extreme temperatures in Denmark, 1994-2010. BMC Infect Dis. 2011; 11:350.

4. Nicholson KG. Clinical features of influenza. Semin Respir Infect. 1992; 7:26–37.
5. Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008; 121:258–264.

7. Studahl M. Influenza virus and CNS manifestations. J Clin Virol. 2003; 28:225–232.

8. Kusznierz G, Uboldi A, Sosa G, Torales S, Colombo J, Moyano C, Escobar H, Lejona S, Anchart E, Gómez A, Imaz S. Clinical features of the hospitalized patients with 2009 pandemic influenza A (H1N1) in Santa Fe, Argentina. Influenza Other Respi Viruses. 2013; 7:410–417.

9. Govaert TM, Dinant GJ, Aretz K, Knottnerus JA. The predictive value of influenza symptomatology in elderly people. Fam Pract. 1998; 15:16–22.

11. Challen K, Goodacre SW, Wilson R, Bentley A, Campbell M, Fitzsimmons C, Walter D. Evaluation of triage methods used to select patients with suspected pandemic influenza for hospital admission. Emerg Med J. 2012; 29:383–388.

12. Koskiniemi M, Rantalaiho T, Piiparinen H, von Bonsdorff CH, Färkkilä M, Järvinen A, Kinnunen E, Koskiniemi S, Mannonen L, Muttilainen M, Linnavuori K, Porras J, Puolakkainen M, Räihä K, Salonen EM, Ukkonen P, Vaheri A, Valtonen V. Study Group. Infections of the central nervous system of suspected viral origin: a collaborative study from Finland. J Neurovirol. 2001; 7:400–408.

13. Kolski H, Ford-Jones EL, Richardson S, Petric M, Nelson S, Jamieson F, Blaser S, Gold R, Otsubo H, Heurter H, MacGregor D. Etiology of acute childhood encephalitis at The Hospital for Sick Children, Toronto, 1994-1995. Clin Infect Dis. 1998; 26:398–409.

14. Guan XR, Li X, Xin XM, Jiang LX, Cui LY, Wang LF, Li HY. Influenza virus infection and risk of acute myocardial infarction. Inflammation. 2008; 31:266–272.

Supplementary Material
Supplementary Table 1
The result of respiratory virus PCR test









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download