Introduction
Corynebacterium species are non-spore-forming aerobic Gram-positive bacilli that are spread widely in the environment and constitute part of the normal flora on skin and mucosa in humans [
12]. In a hospital setting, non-
diphtheriae Corynebacterium are commonly considered contaminants when found in blood cultures because of their low virulence. Recently, there have been increasingly frequent reports of infection by non-
diphtheriae Corynebacterium in immunocompromised patients who were hospitalized or in immunocompetent patients who had implanted medical devices.
Corynebacterium striatum is a commonly-isolated corynebacteria in the clinical microbiology laboratory, although confirmed infections from sterile sites are relatively rare [
3].
C. striatum can cause pulmonary abscess, meningitis, septic arthritis, vertebral osteomyelitis, catheter-related blood stream infection and native- and prosthetic-valve endocarditis [
2345]. The aid of molecular diagnostic methods, especially the 16S rRNA and
rpoB gene sequencing, has greatly improved the ability to detect
C. striatum in clinical specimen [
56]. Here, we report a case of native-valve endocarditis caused by
C. striatum, which was accurately identified by 16S ribosomal RNA sequencing in a non-immunocompromised patient.
Case report
A 55-year-old man with no significant medical history was transferred to our hospital with a 14-day history of fever and lethargy. Over the previous 5 weeks, he had been treated for traumatic subdural hemorrhage after a car accident. Two weeks prior to transfer, a high fever suddenly developed and persisted despite administration of broad-spectrum antibiotics including ceftriaxone, piperacillin-tazobactam, or meropenem in combination with moxifloxacin. There was a healing abrasion on his hand after the car accident, but there were no stigmata of endocarditis. He had no indwelling medical device and no medical history of allergy.
On admission to our hospital, his blood pressure was 150/100 mmHg, pulse rate was regular at 100 beats per minute, and temperature was 38.0°C. He was slightly drowsy and confused but exhibited no neurologic deformity. Heart sounds were regular without murmur, and breathing sounds were normal.
His white blood cell count was 7,400/mm3 with 79.4% neutrophils. Laboratory data were as follows: hemoglobin, 11.2 g/dL; platelets, 251,000/mm3; C-reactive protein, 79.7 mg/dL; procalcitonin 0.18 ng/mL; and erythrocyte sedimentation rate, 52 mm/h. Electrolyte levels and kidney and liver function tests were normal.
Electrocardiogram showed normal sinus rhythm. Computed tomography (CT) of the chest showed pulmonary emphysema, and abdominal CT was normal. Brain CT revealed a small, chronic subdural hematoma on the left frontal convexity.
The patient initially received 2 g ceftazidime intravenously every 8 hours in combination with oral metronidazole (500 mg every 8 hours). On the third day of admission, 2 separate sets of blood cultures were positive for Gram-positive cocci. After the second set of blood cultures, intravenous vancomycin (1 g every 12 h) was added empirically. On the sixth day,
Kocuria kristinae was identified in both blood cultures using the Vitek 2 system (bioMérieux, Marcy-l’Etoile, France) in the absence of definitive laboratory guidelines for determining the antibiotic susceptibility. While the initial blood culture was regarded as contaminated, when the same pathogen was isolated from the consecutive blood culture, we determined that
K. kristinae might be the true pathogen. At this time, initial empiric ceftazidime and metronidazole were discontinued and intravenous vancomycin was maintained. Because
K. kristinae is not a common infective endocarditis pathogen, we also performed 16S rRNA sequencing analysis to confirm the identification of the pathogen in the blood isolates. The universal eubacterial primers fU1 (5’-TTGGAGAGTTTGATCCTGGCTC-3’) and rU2 (GGACTACCAGGGTATCTAA-3’) were used. The 16S rRNA gene sequence (766 bp) was blasted with NCBI Blast website
http://blast.ncbi.nlm.nih.gov/Blast.cgi. The sequence was 99.00% identical to that of
C. striatum was identified (GenBank accession number JF342700.1). Transthoracic echocardiography showed 2 mobile oscillating masses at the tip of the mitral valve leaflet. Transesophageal echocardiography revealed two hypermobile echogenic masses on the anterior and posterior mitral valve leaflets, 10 mm and 8 mm, respectively (
Fig. 1). There was no involvement of either the subvalvular structure or paravalvular structure. There was no evidence of any metastatic lesions at other sites.
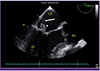 | Figure 1
Transesophageal echocardiogram findings.
Large, hypermobile vegetations were attached to the middle scallop of the anterior (arrowhead) and posterior mitral valve leaflets (arrow), 10 mm and 8 mm, respectively. Vegetation on the posterior mitral valve leaflet showed a 7 mm lineal mobile structure, which indicates a high embolic risk.
LA, left atrium; LV, left ventricle; Ao, aorta.

|
Thus, the patient was diagnosed with C. striatum infective endocarditis not K. kristinae infective endocarditis. After vancomycin was administrated, the fever subsided dramatically. Because of the high embolic risk, mitral valve replacement was performed. Interestingly, K. kristinae, which was found in the blood cultures, was also cultured from mitral valve vegetation despite administration of vancomycin for 14 days. The patient completed 6 weeks of intravenous vancomycin for prosthetic valve endocarditis. At a follow-up over one year later, the patient remained free of infection
Discussion
Non-
diphtheriae Corynebacterium are commonly isolated from clinical specimens but are typically considered contaminants. In addition to
C. diphtheriae,
Corynebacterium are considered organisms that are normal inhabitants of human skin and respiratory tract [
1237]. Also, with the use of molecular diagnostic methods, the taxonomy of
Corynebacterium species has changed and has been reclassified from earlier defined taxa in recent years [
1]. There is an increased frequency of reported non-
diphtheriae Corynebacterium infections, particularly as a cause of nosocomial infection in hospitalized and immunocompromised patients, though these pathogens are widely distributed in the environment and mucous membranes of humans. Common nosocomial pathogens include
Corynebacterium amycolatum, Corynebacterium jeikeium, Corynebacterium urealyticum, and
C. striatum [
8].
C. striatum is widely distributed in the environment, especially in hospital settings associated with nosocomial infection. It colonizes on the skin and mucous membranes of normal hosts and hospitalized patients, and disruption of its integument may lead to bacteremia and subsequent septic complications in either immunocompromised patients or patients with medical devices [
379]. In addition, while
C. striatum is frequently isolated in polymicrobial infections, its degree of pathogenicity is unclear, and differentiation of colonization from pathogen-causing infection has been difficult [
3]. This organism can cause pneumonia, empyema, meningitis, septic arthritis, vertebral osteomyelitis, and endocarditis [
234].
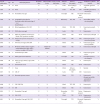
C. striatum is an uncommon cause of infective endocarditis. An English language literature search in Medline revealed 22 previously reported cases of endocarditis due to
C. striatum (
Table 1). The majority of cases (82%, 18/22) are native valve endocarditis. More than half of cases are hospital-acquired infection, and 11 cases (50%) are associated with medical devices, including 4 cases of prosthetic valve, 3 cases of pacemaker lead, 3 cases of vascular access for hemodialysis, and 1 case of ventriculo-atrial shunt. Valve replacements are performed in one quarter of the cases and overall mortality approaches 27%. In our case, the patient had recently experienced a long-term hospital stay and had a scar from an abrasion received during a traffic accident, but had not required a central venous catheter.
Table 1
Reported cases of infective endocarditis caused by Corynebacterium striatum
|
Year of publication |
Age |
Sex |
Underlying disease |
Prosthetic valve |
Other medical device |
Noso-comial risk factors |
Affected valve |
Echocardiography |
Surgery |
Antibiotic (s) administrated |
Survival |
|
1990 |
76 |
M |
None |
N |
N |
N |
Aortic |
Echocardiography |
N |
Ampicillin, gentamicin |
N |
|
1994 |
54 |
M |
None |
N |
N |
N |
Aortic |
TTE, TEE |
AVR |
Penicillin, gentamicin; vancomycin |
Y |
|
1996 |
73 |
M |
Pacemaker (6 yr ago) |
N |
Y |
Y |
Tricuspid |
TEE |
Electrode lead removal |
vancomycin |
Y |
|
1996 |
24 |
M |
Congenital hydrocephalus, Ventriculo-atrial shunt state (age of 2 m) sacral bedsore |
N |
Y |
Y |
Pulmonary |
TTE, TEE |
N |
Amoxicillin, netilmicin, teicoplanin |
Y |
|
2002 |
68 |
M |
Mitral regurgitation, DM, CHF |
N |
N |
N |
Mitral |
TTE, TEE |
N |
Vancomycin; penicillin |
Y |
|
2002 |
62 |
F |
AVR (a few years ago) |
Y |
N |
N |
Aortic |
TEE |
N |
vancomycin |
Y |
|
2002 |
69 |
F |
ESRD via prosthetic arteriovenous fistula, ANCA-positive vasculitis |
N |
Y |
Y |
Mitral |
TEE |
MVR |
Vancomycin, rifampin |
N |
|
2002 |
50 |
M |
Surgery for mycotic aneurysm (2 m ago) |
N |
N |
Y |
Aortic |
TEE |
AVR |
Vancomycin, gentamicin, doxycycline |
Y |
|
2002 |
72 |
F |
AVR state (52 d ago), DM, IHD |
Y |
N |
Y |
Mitral |
TTE |
N |
Vancomycin, gentamicin; penicillin |
N |
|
2005 |
72 |
F |
MVR state (1990), culture-negative endocarditis (18 m ago), ANCA positive vasculitis, ARF on HD |
Y |
Maybe HD catheter |
Y |
Mitral |
TEE |
N |
Vancomycin, rifampin |
Y |
|
2005 |
61 |
F |
Cutaneous lupus, IHD |
N |
N |
N |
Mitral |
TEE |
N |
Vancomycin, gentamicin |
Y |
|
2005 |
46 |
F |
ESRD, graft-related infection |
N |
Y |
Y |
Tricuspid |
TEE |
N |
Linezolid; daptomycin, rifampin |
Y |
|
2006 |
68 |
M |
AVR (3 yr ago), MVR (1y ago), CHF, AF, CVA |
Y |
N |
N |
Mitral |
TTE |
N |
Vancomycin |
Y |
|
2006 |
69 |
F |
Endometrial cancer |
N |
N |
Y |
Mitral |
TEE |
MVR |
Vancomycin |
Y |
|
2006 |
77 |
F |
None |
N |
N |
N |
Mitral |
Echocardiography |
N |
Medical |
Y |
|
2007 |
62 |
M |
CRF, AF |
N |
N |
Y |
Aortic |
TEE |
AVR |
Vancomycin |
Y |
|
2008 |
83 |
M |
Metastatic prostate cancer |
N |
N |
N |
Mitral |
TTE |
N |
Vancomycin, rifampin; penicillin, gentamicin; daptomycin |
N |
|
2008 |
73 |
F |
CHF, CRF, DM |
N |
N |
Y |
Mitral |
TTE, TEE |
N |
Vancomycin |
Y |
|
2009 |
71 |
M |
DM |
N |
N |
Y |
Mitral |
TEE |
N |
Vancomycin |
N |
|
2010 |
71 |
F |
Pacemaker (2 m ago) |
N |
Y |
Y |
Pacemaker lead |
TTE |
Device removal |
Daptomycin |
Y |
|
2012 |
56 |
M |
DM, ESRD |
N |
N |
Y |
Mitral |
TEE |
MVR |
Daptomycin; telavancin |
N |
|
2013 |
78 |
M |
Pacemaker (6 m ago), DM, CRF |
N |
Y |
N |
Tricuspid |
TTE |
Electrode lead removal |
Daptomycin |
Y |

One interesting finding is that
C. striatum can be misidentified as
K. kristinae using automated systems (bioMérieux Vitek 2 GP card). Identification of the genus
Corynebacterium to species level is usually based on biochemical tests. Though API Coryne system is a useful tool for identifying
Corynebacterium species in the clinical laboratory, this may not incorporate all of the tests necessary for the identification of every
Corynebacterium species. In recent years, the introduction of molecular methods, especially 16S rRNA gene and
rpoB gene sequencing, has improved the ability to identification of
Corynebacterium species [
5610]. In our case, the causative organism was misidentified as
K. kristinae by the commercial identification kit but was confirmed as
C. striatum by 16S rRNA sequence analysis. There is a previous similar case report of misidentification of
C. striatum as
K. kristinae using the commercial identification kit [
11]. In literature review, there are only 3 cases of infective endocarditis caused by
C. striatum identified by 16S rRNA sequencing [
101213]. Clinically, when organisms such as
C. striatum or
K. kristinae are identified from subsequent blood cultures in unusual clinical scenarios, we suggest a genotypic assay, such as 16S rRNA, to confirm species identity. Additionally, 16S rRNA sequencing of resected endocardial specimen is useful tool for verifying the causative agent [
1415]. Bosshard
et al. [
14] suggested that the sensitivity, specificity, and positive and negative predictive values of 16S rRNA sequencing were 94%, 100%, 100%, and 90%, for cases of native valve endocarditis. In our case, unfortunately, 16S rRNA sequencing on vegetation acquired in the operative field was not performed. However, because organisms detected from blood and vegetation were both misidentified as
K. kristinae using the commercial identification kit, the microorganisms in the vegetation might be
C. striatum. Despite the excellent specificity of 16S rRNA sequencing, clinicians have to be aware of the interpretation of a positive result within clinical context; even several months after completion of successful therapy for endocarditis, 16S rRNA sequencing results may still be positive [
14].
Antibiotic-susceptibility data of
C. striatum is scarce. However,
C. striatum may be susceptible to vancomycin but resistant to penicillin, ciprofloxacin, erythromycin, rifampin, and tetracycline; it has variable susceptibility to other β-lactams and aminoglycosides [
2]. Recently, the emergence of multidrug-resistant strains acting as nosocomial pathogens was reported in long-term hospitalized patients with underlying disease, and the most effective antibiotic was vancomycin [
371316]. There are no definitive laboratory guidelines for determining the antibiotic susceptibility of coryneform bacteria, and in our case, susceptibility testing was not available. We treated the patient with vancomycin, which was reported as the most active antibiotic against corynebacteria in the literature, and clinical response was good. Defervescence was achieved after 3 days of vancomycin treatment. In a literature review of
C. striatum endocarditis, there were more cases treated with medical therapy alone than with valve replacement (
Table 1). In our case, we performed mitral valve replacement therapy due to high embolic risk. Interestingly, resected vegetation was culture-positive despite 14 days of vancomycin treatment, so we administrated antibiotics for another 4 weeks after valve resection. This bacterial persistence might be associated with multidrug resistance or a high tolerance to antibiotic-induced killing in
C. striatum. According to Yoo
et al. [
16], we need awareness of the emergence of multidrug-resistant
C. striatum in Korea; however, further investigation is required.
Because Corynebacterium species are usually considered to be contaminants and are not routinely identified to the species level, C. striatum infection rates are probably underestimated. In unusual clinical scenarios, blood cultures positive for Corynebacterium species or K. kristinae, as determined by a commercial automatic culture system, should not be overlooked. The possibility of C. striatum infective endocarditis should be considered even in a patient without structural heart disease or prosthetic valves, and the genotypic assay, such as 16S rRNA sequence analysis, may be very useful.






 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download