Abstract
Staphylococcus saprophyticus is a common pathogen of acute urinary tract infection (UTI) in young females. However, S. saprophyticus bacteremia originating from UTI is very rare and has not been reported in Korea. We report a case of S. saprophyticus bacteremia from UTI in a 60-year-old female with a urinary stone treated successfully with intravenous ciprofloxacin, and review the cases of S. saprophyticus bacteremia reported in the literature. Thus, the microorganism may cause invasive infection and should be considered when S. saprophyticus is isolated from blood cultures in patients with UTI.
Staphylococcus saprophyticus is a Gram-positive, coagulase-negative species of Staphylococcus [1] and a major uropathogen of uncomplicated urinary tract infection (UTI), accounting for up to 42% of UTIs in young females [2]. However, the clinical significance of this organism isolated blood culture has not been understood well. In a study on the clinical significance of S. saprophyticus bacteremia, the most common portal of entry was central venous catheter in immunocom-promised patients [3]. However, S. saprophyticus bacteremia originating from UTI is very rare and has not been reported in Korea. Herein, we report the first case of S. saprophyticus bacteremia originating from UTI in Korea and we discuss the reason why the frequency of S. saprophyticus bacteremic UTI is low.
A 60-year-old female was admitted to the emergency department with chest pain from the previous day. She had a fever of 38.6℃ accompanied by left flank pain after 4 h of admission to the emergency department. Her past medical history included 7 years of controlled type 2 diabetes mellitus treated with oral hypoglycemic drugs. She was diagnosed with angina 5 years prior to this admission and had percutaneous coronary intervention.
On examination, the patient's body temperature was 38.6℃ and blood pressure was 160/90 mmHg. Heart rate and oxygen saturation were 73 beats/min and 95% in room air. She was alert and left-sided costovertebral angle tenderness was noted. An electrocardiogram showed a depression of the ST segment in V4-V6 and left ventricular hypertrophy with strain. Her troponin I and CK-MB were elevated to 2.57 ng/L and 18.1 ng/mL, respectively. Laboratory data showed her white cell count was elevated at 15.24 × 109/L (normal 4.0-10.0 × 109/L). Blood glucose level was 187 mg/dL (normal 70-110 mg/dL). Blood urea nitrogen and creatinine were normal at 15.3 mg/dL (normal 6-20 mg/dL) and 0.62 mg/dL (normal 0.5-0.9 mg/dL), respectively. Her C-reactive protein level was 6.8 mg/L (normal 0-5 mg/L). Urinalysis showed a pH of 7, negative nitrates, and 20-29 white blood cells/high power field. The computed tomography (CT) scan of the patient's abdomen revealed an obstructive 6-mm-sized left renal pelvic stone, with left hydroureteronephrosis and proximal ureter wall thickening (Fig. 1).
The patient was transferred to the Department of Cardiology with non-ST-segment elevation myocardial infarction and UTI. Antiplatelet agents were administered and intravenous antibiotic therapy was initiated with ciprofloxacin. Urine culture from the patient's original emergency department visit revealed methicillin-resistant S. saprophyticus of >100,000 colony-forming units/mL.
Blood culture grew Gram-positive cocci in both aerobic and anaerobic bottles in four out of four sets. Blood culture (Vitek-2; Biomérieux, Durham, NC, USA) isolation revealed methicillin-resistant S. saprophyticus, which was confirmed by 16S rDNA sequencing. Antibiotic susceptibility testing (Vitek-2; Biomérieux, Durham, NC, USA) for the following antimicrobial agents revealed minimum inhibitory concentrations (MIC) as follows: ciprofloxacin (MIC ≤ 0.5 µg/mL, susceptible), penicillin (MIC ≥ 0.5 µg/mL, resistant), oxacillin (MIC ≥ 4 µg/mL, resistant), and vancomycin (MIC = 1 µg/mL, susceptible). According to the antibiotic susceptibility of S. saprophyticus, the patient was treated with intravenous ciprofloxacin. Follow-up urine culture was carried out 4 days after commencement of antimicrobial therapy and the result was negative. The patient's condition improved with 7 days of ciprofloxacin treatment. She was discharged from hospital with 1-month outpatient follow up for left renal pelvis stone and prescription of antiplatelets with atorvastatin. During outpatient follow up, potassium citrate was administered and the left renal pelvis stone had disappeared with improving hydronephrosis at the 2-month follow up CT scan.
S. saprophyticus is the second most common uropathogen after Escherichia coli in uncomplicated UTI in females [4]. A nationwide surveillance study of uncomplicated UTI reported that S. saprophyticus (5.2%) was the second most common uropathogen in the total population, and significantly more common in premenopausal females [5]. Similarly, another study reported that the most common uropathogens were E. coli (53.3%) and S. saprophyticus (2.5%) among 4,734 females with uncomplicated UTI in multi-community centers [6]. A Korean study of 24,277 urinary specimen strains from 1996 to 2008 showed that the most common pathogen was E. coli (23.8%), followed by Enterococcus faecalis (11.0%), Enterococcus faecium (10.8%), Pseudomonas aeruginosa (9.0%), coagulase negative staphylococci (7.7%), Klebsiella pneumoniae (6.6%), Staphylococcus aureus (3.3%), S. saprophyticus (0.1%) [4]. Contrary to previous foreign studies, the isolation of S. saprophyticus was much less frequent from UTI patients in Korea. Because S. saprophyticus is a pathogen of uncomplicated UTI in young women, the isolation rate of the S. saprophyticus may be lower in admitted patients. Therefore, relatively few patients with S. saprophyticus UTI may be included because reports on surveillance for UTI pathogens were performed at tertiary hospitals in Korea [234].
In a case series of patients with S. saprophyticus bacteremia, there have been no reports of S. saprophyticus bacteremia originating from UTI in Korea to date, and most bacteremias were associated with tunneled-central venous catheters in patients with immune suppression, such as hematologic malignancies [3]. As shown in Table 1 [7891011], three patients had S. saprophyticus bacteremia from UTIs combined with nephrolithiasis, and four patients were healthy young females with a history of intense sexual activity prior to admission. Two patients had pregnancy as a clinical setting. All of these patients were successfully treated with intravenous antibiotics.
We speculate why S. saprophyticus bacteremia was rare, although UTI caused by S. saprophyticus was common. First, S. saprophyticus did not produce coagulase, unlike other staphylococci such as S. aureus. Coagulase converts fibrinogen to fibrin and reacts with prothrombin in blood. This reaction results in blood clotting, coating the bacterial cell surface with fibrin and enabling the staphylococci to resist phagocytosis. In this sense, it is proposed that S. saprophyticus has lower virulence than S. aureus, especially in blood. Second, S. saprophyticus did not possess a potassium acquisition system such as ATPase, which is necessary for bacterial growth [12]. A potassium-plentiful environment such as urine enables S. saprophyticus growth without a potassium-import system [12], whereas S. saprophyticus has difficulty of bacterial growth in environments of relatively low potassium concentration such as blood. Third, S. saprophyticus has cell wall-anchoring adhesins to the urinary tract epithelium as a virulence factor, but do not possess the extracellular matrix-binding proteins that are found in S. aureus or Staphylococcus epidermidis and enable S. aureus or S. epidermidis to anchor to the peptidoglycan of extracellular matrix [12].
In this report, the patient had an obstructing stone on her left renal pelvis with hydroureteronephrosis. We postulated that urinary tract obstruction by a renal stone enabled S. saprophyticus to reach the renal pelvis easily, and aggravated the tissue invasiveness of bacteria from the urinary tract. Although S. saprophyticus bacteremia from UTI was rare, obstructive nephrolithiasis seems to be a predisposing factor for bacteremia from UTI [9]. Pregnancy can also play a role in functional obstruction and impedance of the descending flow of urine [13].
This case is the first report of S. saprophyticus bacteremia from UTI in Korea. We suggest that S. saprophyticus UTI may result in bacteremia in patients with nephrolithiasis or any cause of obstruction. Thus, S. saprophyticus may cause invasive infection and should be considered when this microbe is isolated from blood cultures in patients with UTI, especially in patients with urinary tract obstruction due to urinary stones or pregnancy.
Figures and Tables
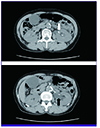 | Figure 1An obstructing 6-mm-sized renal stone (white arrow) on the pelvis of the left kidney, with hydroureteronephrosis (black arrow), and proximal ureter wall thickening on abdominal computed tomography scan. |
Table 1
Summary of previous reports of Staphylococcus saprophyticus bacteremia from urinary tract infections.

| Case | Year | Sex/Age | Underlying clinical factors | Probable source of bacteremia | Therapy | Outcome (cause of death) |
|---|---|---|---|---|---|---|
| Present case | 2012 | F/60 | Type 2 diabetes mellitus, angina, renal stone | Urinary tract infection | Ciprofloxacin | Recovered |
| Golledge [7] | 1988 | F/14 | Sexual activity | Urinary tract infection | Amoxicillin, cloxacillin, penicillin | Recovered |
| Golledge [7] | 1988 | F/49 | Sexual activity | Urinary tract infection | Cephalothin, gentamicin, penicillin | Recovered |
| Glimaker [8] | 1988 | F/19 | Sexual activity | Urinary tract infection | Co-trimoxazole, cloxacillin, flucloxacillin | Recovered |
| Glimaker [8] | 1988 | F/33 | Ureteric calculus Sexual activity | Urinary tract infection | Co-trimoxazole | Recovered |
| Olafsen [9] | 1986 | F/27 | Previous pyelonephritis, Pregnancy, ureteric calculus | Urinary tract infection | Ampicillin, amoxicillin | Recovered |
| Chen [10] | 2014 | F/38 | Unknown | Urinary tract infection | Gentamicin | Recovered |
| Lee [11] | 1987 | F/38 | Pregnancy | Urinary tract infection | Cefazolin | Recovered |
References
1. Schleifer KH, Kloos WE. Isolation and characterization of Staphylococci from human skin I. amended descriptions of Staphylococcus epidermidis and Staphylococcus saprophyticus and descriptions of three new species: Staphylococcus cohnii, Staphylococcus haemolyticus, and Staphylococcus xylosus. Int J Sys Evol Microbiol. 1975; 25:50–61.
2. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med. 2002; 113:Suppl 1A. 5S–13S.
3. Choi SH, Woo JH, Jeong JY, Kim NJ, Kim MN, Kim YS, Ryu J. Clinical significance of Staphylococcus saprophyticus identified on blood culture in a tertiary care hospital. Diagn Microbiol Infect Dis. 2006; 56:337–339.
4. Kim SJ, Kwon O, Uh Y, Hwang GY, Jang IH, Yoon KJ, Kim HY. Frequency and clinical characteristics of urinary tract infections caused by Staphylococcus saprophyticus. Korean J Clin Microbiol. 2009; 12:62–66.

5. Hayami H, Takahashi S, Ishikawa K, Yasuda M, Yamamoto S, Uehara S, Hamasuna R, Matsumoto T, Minamitani S, Watanabe A, Iwamoto A, Totsuka K, Kadota J, Sunakawa K, Sato J, Hanaki H, Tsukamoto T, Kiyota H, Egawa S, Tanaka K, Arakawa S, Fujisawa M, Kumon H, Kobayashi K, Matsubara A, Naito S, Tatsugami K, Yamaguchi T, Ito S, Kanokogi M, Narita H, Kawano H, Hosobe T, Takayama K, Sumii T, Fujii A, Sato T, Yamauchi T, Izumitani M, Chokyu H, Ihara H, Akiyama K, Yoshioka M, Uno S, Monden K, Kano M, Kaji S, Kawai S, Ito K, Inatomi H, Nishimura H, Ikuyama T, Nishi S, Takahashi K, Kawano Y, Ishihara S, Tsuneyoshi K, Matsushita S, Yamane T, Hirose T, Fujihiro S, Endo K, Oka Y, Takeyama K, Kimura T, Uemura T. Nationwide surveillance of bacterial pathogens from patients with acute uncomplicated cystitis conducted by the Japanese surveillance committee during 2009 and 2010: Antimicrobial susceptibility of Escherichia coli and Staphylococcus saprophyticus. J Infect Chemother. 2013; 19:393–403.

6. Kahlmeter G. ECO SENS. An international survey of the antimicrobial susceptibility of pathogens from uncomplicated urinary tract infections: the ECO.SENS Project. J Antimicrob Chemother. 2003; 51:69–76.

8. Glimaker M, Granert C, Krook A. Septicemia caused by Staphylococcus saprophyticus. Scand J Infect Dis. 1988; 20:347–348.
9. Olafsen LD, Melby K. Urinary tract infection with septicaemia due to Staphylococcus saprophyticus in a patient with a ureteric calculus. J Infect. 1986; 13:92–93.
10. Chen CH. Staphylococcus saprophyticus bacteremia with pyelonephritis cured by gentamicin. J Formos Med Assoc. 2014; 113:483–484.

11. Lee W, Carpenter RJ, Phillips LE, Faro S. Pyelonephritis and sepsis due to Staphylococcus saprophyticus. J Infect Dis. 1987; 155:1079–1080.

12. Kuroda M, Yamashita A, Hirakawa H, Kumano M, Morikawa K, Higashide M, Maruyama A, Inose Y, Matoba K, Toh H, Kuhara S, Hattori M, Ohta T. Whole genome sequence of Staphylococcus saprophyticus reveals the pathogenesis of uncomplicated urinary tract infection. Proc Natl Acad Sci U S A. 2005; 102:13272–13277.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download