Abstract
The incidence of human immunodeficiency virus (HIV) infections continue to increase throughout the world. Although neurologic complications are frequent in individuals with HIV infection or acquired immunodeficiency syndrome (AIDS), vestibulocochlear neuritis is still a relatively rare manifestation. We report the first case of vestibulocochlear neuritis occurring in an AIDS patient in Korea.
Worldwide, the incidence and prevalence of acquired immunodeficiency syndrome (AIDS) are on the rise. Neurologic manifestations are particularly common in individuals with AIDS, and ear, nose, and throat manifestations are frequently the earliest symptoms of immunodeficiency. Such manifestations are caused either by human immunodeficiency virus (HIV) itself or by opportunistic infections. However, sudden sensorineural hearing loss (SSHL) due to vestibulocochlear neuritis has rarely been reported [1]. In the literature, there are a few case reports describing vestibulocochlear neuritis in patients with AIDS, including two cases associated with HIV seroconversion syndrome [12], one case due to cerebral lymphocytic infiltration [3], two cases caused by cytomegalovirus (CMV) infection [4], and two cases with otosyphilis [56]. Herein, we report the first case of vestibulocochlear neuritis in an AIDS patient in Korea.
A 43-year-old-man, who had been HIV seropositive for 10 years, was admitted with a 4-week history of sudden bilateral hearing loss and tinnitus. He had a 30 pack-year history of smoking.
The patient had been diagnosed with HIV infection 10 years ago, after an unprotected sexual intercourse in Cambodia. He had initially presented with CMV colitis, accompanied by 20 kg of weight loss, 3 years after the diagnosis of HIV infection. He was treated with valganciclovir and the antiretroviral drugs zidovudine/lamivudine (ZDV/3TC) and lopinavir/ritonavir (LPVr). The patient reported that he was compliant with his antiretroviral medications, and remained free of symptoms for 5 years, until he developed herpes zoster, with his HIV RNA level 41,000 copies/mL. Early last year, the patient presented with onychomycosis and Pneumocystis jirovecii pneumonia. His HIV RNA levels had remained similarly high (29,300 copies/mL) until last October, when the antiretroviral regimen was changed to ZDV/3TC, ritonavir (RTV), and atazanavir (ATV). Afterwards, his serum HIV RNA levels remained consistently low (<20 copies/mL). One month prior to admission, the patient began to suffer from acute hearing loss and dizziness. The hearing loss worsened progressively despite treatment from a local otolaryngology clinic. Initially, the patient had difficulty perceiving high-pitched sounds; later, he had trouble comprehending normal speech. Four days before his admission, the patient developed severe dyspnea, which prompted him to seek medical care.
The patient's initial vital signs included a body temperature of 36.5℃, blood pressure of 106/50 mmHg, heart rate of 84/min, and respiratory rate of 24/min. The patient appeared to be acutely ill. Upon physical examination, the patient was found to have severe peripheral muscle wasting and general weakness. His height was 170 cm; weight, 55 kg; and body mass index, 19.03. Auscultation of the chest revealed bilateral basilar rales.
Laboratory examination revealed an elevated C-reactive protein level of 17.63 mg/dL. White blood cell count was 14,370/mm3, with 84.1% neutrophils, 9.7% lymphocytes, and 3.6% monocytes. Other abnormal initial laboratory results included the following: lactate dehydrogenase, 494 IU/L; C-reactive protein, 17.63 mg/dL; activated partial throboplastin time, 45.5 seconds; D-dimer, 1.72 µg/mL; serum creatinine, 0.63 mg/dL; serum albumin, 4.2 g/dL; alkaline phosphatase, 104 IU/L; total bilirubin, 2.93 mg/dL; hemoglobin, 11.9 g/dL; mean corpuscular volume, 110.7 fl; and sodium, 130 mEq/L. His CD4+ cell count was 82 cells/mm3, and serum HIV RNA level was <20 copies/mL. Although antiretroviral drug resistance could not be measured owing to low serum HIV RNA titer, results of the assay conducted the previous year did not reveal any drug resistance. Polymerase chain reaction (PCR) for Mycobacterium. tuberculosis was negative, and so were results of serological tests for influenza antigen, HBs antigen, HBs antibody, HCV antibody, cold agglutinin, Toxoplasma IgM and IgG antibodies, and Mycoplasma pneumoniae IgM antibody. Anaerobic cultures of blood displayed no growth for 5 days, whilst sputum culture was positive for Candida albicans. Serum polymerase chain reaction for P. jirovecii was negative.
Although the patient's pulmonary symptoms resolved after 6 days of trimethoprim-sulfamethoxazole, bilateral SSHL and dizziness persisted. Cerebrospinal fluid analysis showed a cell count of 30 cells/µL (mostly lymphocytes), pH of 8.0, glucose level of 59 mg/dL, and total protein level of 110.3 mg/dL. PCR for CMV and John Cunningham (JC) virus in the cerebrospinal fluid were negative; results of serological tests for syphilis and Cryptococcus neoformans antigens also displayed negative results.
Auditory function tests confirmed the presence of SSHL on both sides. There were bilateral losses of pure-tone discrimination mainly in the high frequency range. Pure-tone averages (PTA) indicated mild hearing loss in the right ear (PTA = 50 dB) and moderate hearing loss in the left ear (PTA = 56 dB). Brainstem auditory evoked responses (BAER) showed borderline I-III interwave intervals (left ear = 2.32 ms, right ear = 2.36 ms) and prolonged I-V interwave intervals (left = 4.62 ms, right = 4.46 ms) in both ears. Delayed latencies of wave V in BAER suggested neuropathies of the central auditory and vestibular regions [7]. In the vestibular function tests, a mild asymmetry was observed.
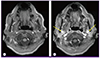
Brain magnetic resonance imaging with gadolinium enhancement showed contrast uptake by both vestibulocochlear nerves in the internal acoustic canals, suggestive of vestibulocochlear neuritis (Fig. 1). Methylprednisolone, 24 mg/day, was started. A week later, there was an improvement in the pure-tone averages (left ear = 38 dB, right ear = 36 dB), normalization of BAER latencies, and disappearance of tinnitus.
The patient's hearing loss improved after 7 days of glucocorticoid use, but vestibular dysfunction persisted and manifested as a mild asymmetry on vestibular function tests. After 3 days, the patient no longer complained of dizziness, and he was discharged. At the 1-month follow-up, he was free of all otologic symptoms.
SSHL is often caused by tumors, head trauma, and/or ototoxic drug use. Other etiologic causes implicated in SSHL include viral infections, vasculitis, and autoimmune diseases. However, it is not commonly associated with HIV [1].
The otologic symptoms in AIDS patients may result from a combination of the effects of HIV infection coupled with opportunistic microorganisms, or the possible ototoxic effects of certain chemotherapeutic agents [8]. Our patient experienced a sudden onset of bilateral SSHL due to vestibulocochlear neuritis in AIDS. He had neither a history of head trauma nor ototoxic drug use, although heavy smoking could have contributed to the appearance of SSHL. There were clinical and neurophysiological evidences of bilateral cochlear nerve involvement, as shown by the presence of bilateral SSHL in the high frequency ranges on pure-tone audiograms and abnormally delayed latencies on brainstem auditory evoked responses. On the other hand, vestibular involvement was minor, with mild asymmetry seen in the vestibular function tests and absence of clinical features such as nystagmus or ataxia. Attempts were made to elicit the presence of possible etiologic agents by CSF analysis, showing negative results for infections with CMV, JC virus, Treponema pallidum, and C. neoformans infections. Because the patient had a low CD4+ T cell level (82 cells/µL), there was a possibility that reactivation of neurotropic viruses, such as HIV, CMV, or measles virus in the inner ear, triggered inflammatory demyelination of the vestibulocochlear nerves. The exact mechanism involving neuropathies in viral infections is still unknown, but bilateral involvement is thought to be more suggestive of cranial neuropathies due to primary retroviral infections [1]. Furthermore, several reports have proposed that HIV may directly affect the auditory-vestibular pathway [9]. Other etiologic candidates are viruses that cause parainfectious neuritis. Heinze et al. proposed that the advanced stage of HIV infections is associated with a higher prevalence of vestibulocochlear nerve dysfunction [9], which may result from accelerated biological aging caused by HIV [8]. When all possible causes are excluded, idiopathic causes can also be considered.
Additional possible causes of vestibulocochlear neuritis include vasculitis, which has been implicated in other progressive sensorineural deafness syndromes [110]. Also, the uneven distribution of local ischemia seen in vasculitis [1] may account for the less severe involvement of the vestibular branches of the eighth cranial nerve compared to the cochlear branches in our patient. Moreover, the rapid improvement of the hypoacusis with high-dose methylprednisolone therapy suggests a transient inflammatory reaction rather than direct neuronal injury.
It has been suggested that highly active antiretroviral therapy (HAART) itself, including ZDV/3TC, is ototoxic [11]. However, this is less likely in this patient because he had been on ZDV/3TC for more than 7 years without any otologic manifestations. The sudden onset of vestibulocochlear neuritis and its responsiveness to glucocorticoid therapy is more suggestive of an infectious, immunologic or idiopathic cause.
In treating seemingly idiopathic or infectious vestibulocochlear neuritis in HIV-positive patients, steroids should be used with caution, as high-dose steroids can impair the immune function further and result in serious opportunistic infections. For instance, steroid use has been associated with increased incidence of fungal infections such as aspergillosis. To limit such complications, short-term use is advocated.
Otologic manifestations will continue to increase with prolonged survival of AIDS patients. Unlike in patients negative for HIV, opportunistic infections and HIV itself must be considered in the etiologic evaluation of SSHL, or vestibular neuritis in patients with HIV infection. When SSHL arises in immunocompromised patients, they should undergo evaluations for possibly severe causes of SSHL such as CMV infection, syphilis, or schwannoma [3]. In the absence of opportunistic infections, early initiation of therapy with high-dose glucocorticoid could improve the neurologic outcome and quality of life in AIDS patients. As SSHL may be the initial manifestation of HIV in otherwise asymptomatic patients, it is recommended that they undergo testing for HIV [17]. Conversely, routine evaluations of audiological and vestibular functions can be beneficial for patients with AIDS.
Figures and Tables
References
1. Grimaldi LM, Luzi L, Martino GV, Furlan R, Nemni R, Antonelli A, Canal N, Pozza G. Bilateral eighth cranial nerve neuropathy in human immunodeficiency virus infection. J Neurol. 1993; 240:363–366.

2. Gállego-Pérez-Larraya J, Riverol M. Facial diplegia and vestibular neuritis secondary to HIV seroconversion syndrome. Can J Neurol Sci. 2009; 36:527–528.

3. Wenzel GI, Götz F, Lenarz T, Stöver T. HIV-associated cerebral lymphocyte infiltration mimicking vestibular schwannoma. Eur Arch Otorhinolaryngol. 2008; 265:1567–1571.

4. Meynard JL, el Amrani M, Meyohas MC, Fligny I, Gozlan J, Rozenbaum W, Roullet E, Frottier J. Two cases of cytomegalovirus infection revealed by hearing loss in HIV-infected patients. Biomed Pharmacother. 1997; 51:461–463.

5. Chan SY, Medhi M, Ekbote A, Moses S, Sibtain N, Andrews T, O'Connor AF, Kulasegaram R. Syphilis causing hearing loss. Int J STD AIDS. 2008; 19:721–722.

6. Rodríguez-Uña I, Serrador-García M, Santos-Bueso E, Díaz-Valle D, García-Feijóo J. Simultaneous optic and vestibulocochlear syphilitic neuropathy in a patient with HIV infection. J Ophthalmic Inflamm Infect. 2013; 3:27.

7. Rarey KE. Otologic pathophysiology in patients with human immunodeficiency virus. Am J Otolaryngol. 1990; 11:366–369.

8. Lin C, Lin SW, Weng SF, Lin YS. Increased risk of sudden sensorineural hearing loss in patients with human immunodeficiency virus aged 18 to 35 years: a population-based cohort study. JAMA Otolaryngol Head Neck Surg. 2013; 139:251–255.

9. Heinze BM, Vinck BM, Hofmeyer LM, Swanepoel de W. Vestibular involvement in adults with HIV/AIDS. Auris Nasus Larynx. 2014; 41:160–168.

10. Rauch SD. Clinical practice. Idiopathic sudden sensorineural hearing loss. N Engl J Med. 2008; 359:833–840.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download