Abstract
Kikuchi-Fujimoto disease (KFD) is a benign, self-limiting disease, with a specific histopathology. It can be diagnosed clinically, and specific symptoms include fever and cervical lymphadenopathy. The histological finding of KFD in cervical lymph nodes includes necrotizing lymphadenitis. KFD needs conservative treatments. If KFD persists for a long period, steroids or nonsteroidal antiinflammatory drugs can be used to control symptoms. Previous studies have reported the treatment of KFD with hydroxychloroquine (HC) in patients unresponsive to steroids. Herein, we report a case of a 25-year-old female patient diagnosed with KFD unresponsive to steroids, and was successfully treated with HC.
Kikuchi-Fujimoto disease (KFD) was initially diagnosed by the Japanese pathologists Kikuchi and Fujimoto [12]. KFD is a lymphadenitis that invades the cervical lymph nodes. It usually affects Asian women aged 20 to 30 years. The most common symptoms are fever, cervical lymph node enlargement, and pain. Non-specific symptoms including chills, fatigue, diaphoresis, weight loss, joint pain, nausea, and diarrhea can also be present [3].
There are no specific diagnostic tests for KFD. It is diagnosed clinically when cervical lymph node enlargement, pain, and fever occur, along with the diagnosis of histiocytic necrotizing lymphadenitis on lymph node biopsy. The recurrence rate of KFD is 3%-4%, which is low [3]. Non-steroidal anti-inflammatory drugs (NSAIDs) and steroids are used to manage systemic symptoms such as fever and pain.
We report a case of a patient with recurring fever and cervical lymph node enlargement who was diagnosed with KFD and treated with hydroxychloroquine (HC), considering the treatment failure with steroids.
A 25-year-old woman was admitted to the hospital because of gradual lymph node enlargement in the left cervical region, starting 3 days ago.
Five years earlier, the patient was admitted to the hospital because of this same symptom, and was diagnosed with KFD after an excisional biopsy. The patient was treated with NSAIDs and prednisolone at 0.5 mg/kg/day for 2 weeks, and as symptoms resolved, prednisolone dosage was reduced to 0.1 mg/kg/day and then maintained for 2 months for symptom management. However, because the symptoms recurred after this period, another biopsy was performed and the patient was rediagnosed with KFD. It was a second event. At this instance, NSAIDs and prednisolone at 0.4 mg/kg/day were used as conservative treatment.
Three years after hospital discharge, the patient was readmitted because of cervical lymph node enlargement and fever, and after another biopsy, she was rediagnosed with third KFD. NSAIDs and prednisolone at 0.5 mg/kg/day were used as treatment for 3 weeks, and as symptoms resolved, the dosage of prednisolone was decreased. Two years later, the patient was readmitted owing to the same symptoms and was rediagnosed with fourth KFD, at which time the symptoms were managed with NSAIDs.
Vital signs were as follows: blood pressure: 100/60 mmHg, heart rate: 78 bpm, respiratory rate: 18 breaths/min, body temperature: 37.2℃, and mental status: alert. Lymph node enlargement (1.5 × 1.0 cm) with redness was observed, and the patient experienced pain near the left sternocleidomastoid (SCM) muscle, inferior to the left mandible. Axillary lymph nodes and inguinal lymph nodes are not tendered. Otherwise, the physical exam was unremarkable. Peripheral blood test results were as follows: white blood cell count: 5,520/mm3 (44.2% neutrophils), hemoglobin level: 13.5 g/dL, hematocrit: 39.2%, platelet count: 210,000/mm3, prothrombin time: 10.5 s, and activated partial thromboplastin time: 28.3 s. Liver and kidney function test results were unremarkable. Other laboratory test results were as follows: C-reactive protein level: 0.06 mg/dL, antinuclear antibody (ANA) test: positive (1:80), anti-Smith antibody: negative; anti-dsDNA antibody: negative, antiphospholipid antibody: negative, C3 level: 103 mg/dL, C4 level: 30.6 mg/dL, human immunodeficiency virus (HIV) antibody: negative, venereal disease research laboratory antibody: negative, Toxoplasma Imunoglobulin M (Ig M) antibody: negative, cytomegalovirus Ig M antibody: negative, Epstein-Barr virus Ig M antibody: negative. On neck computed tomography, multiple cervical lymph node enlargements (levels II, III, IV, Va, and Vb) were observed (Fig. 1).
While the patient was on intravenous systemic anesthesia, the lymph nodes (1.5 × 0.8 × 0.7 cm) located between the SCM and jugular vein were biopsied by making a 4 cm incision at the level of the hyoid bone at the anterior border of the SCM. Cervical lymph nodes were grossly pale and soft (Fig. 2). Necrotizing lymphadenitis was observed as well as many histiocytes with cell fragments; however, no neutrophil infiltration was observed on hematoxylin and eosin stain. Abundant karyorrhectic debris, which can distort the nodal architecture was observed (Fig. 3). We performed nested PCR for Mycobacterium. tuberculosis. The result was negative.
After the biopsy, the patient presented with symptoms compatible with KFD and was diagnosed with recurrent KFD. Her clinical symptoms did not meet the diagnostic criteria of SLE, despite of ANA positivity. Fever, cervical lymph node enlargement, and pain were managed with NSAIDs and oral prednisolone at 0.5 mg/kg/day for 2 weeks. However, treatment was changed to intravenous methylprednisolone at 1.0 mg/kg/day because fever and lymph node enlargement did not resolve. After 7 days since we started intravenous methylprednisolone, the symptoms still persisted. So we administered HC at 3 mg/kg/day, decreasing the dosage of steroids, and eventually discontinued the use of steroids. Because fever and pain resolved, treatment was continued with HC alone. The patient was discharged from the hospital and was observed at the outpatient clinic with continuation of HC for 4 months.
KFD is a benign disease that affects subjects aged between 20 and 30 years, and is characterized by histiocytic necrotizing lymphadenitis in the cervical lymph nodes. No specific diagnostic methods are available for KFD, and diagnosis is established only by the presence of slowly progressing cervical lymph node enlargement due to lymphadenitis. The mean duration of symptoms varies between 1 and 4 months [4].
Recurrence of KFD is rare and varies between 3% and 7% [3]. However, according to a study involving Korean patients, 20.6% adult patients [3] and 42.4% pediatric patients suffer recurrence [5], and this rate is higher than that observed in other countries. In addition, the onset of recurrence is within several weeks after the initial diagnosis, but is different from that reported in other countries. This seems to be due to the selection bias because patients enrolled in this study were from a Kikuchi center of the tertiary hospital which has patient with more severe symptoms and higher recurrence rate [3]. The site of recurrence is usually the same as the first site. The difference in the severity of the initial symptoms or the method of initial treatment does not lead to differences in the recurrence rate. However, there has been a higher recurrence rate in patients with non-classic symptoms. Classic symptoms are fever, cough, fatigue, and lymph node enlargement. Patients with positive ANA also showed a higher recurrence rate [35]. We think that the reason for higher recurrence rate in pediatric patient is that because KFD in young age is more related to autoimmune reaction and immune dysregulation than that of adult KFD [5]. Comparison of European cases and cases from Asia, Asian patients have higher ANA positivity than Europeans. ANA positivity was risk factor of recurrent KFD and associated with SLE. For better understanding about the recurrence rate of KFD and the recurrent time of KFD in Korean patients further study with variable type of institutions enrolled seems necessary [6].
Even though KFD recurrence is possible, the potential recurrence of KFD must be distinguished from that of tuberculosis, systemic lupus erythematosus (SLE), and lymphoma, among others [7]. It is difficult to distinguish these diseases based solely on the symptoms, and therefore, the performance of tissue biopsy and immuno-serology is necessary. In addition, we can assess the infiltration of other cell types. Reed-Sternberg cells, hematoxylin bodies, and neutrophils are associated with lymphomas, whereas plasma cell infiltrations are associated with SLE. Acid-fast staining can be used for the detection of tuberculosis [789]. Furthermore, SLE and KFD can be differentiated with immuno-serologic tests comprising ANA, anti-phospholipid, and C3 and C4 complement tests, among others [71011].
KFD is characterized by T-cell mediated cytotoxicity which induces apoptosis. Mainly CD8+ cytotoxic T cells lead to apoptosis, and proliferation in KFD [12]. Serum interferon (IFN)-γ has a major role in cell-mediated immunity. Elevated IFN-γ and apoptotic CD123+ plasmacytoid monocytes are reported in KFD. Because of this mechanism, KFD is a self-limiting disease and treatment is not mandatory, in which case NSAIDs can be used as conservative treatment. Accumulation of apoptotic plasmacytoid monocytes in KFD shows parallels with defective clearance of apoptotic cells in SLE and supports a pathophysiologic association [13].
KFD patients can suffer classic symptoms, which include fever, fatigue, and cervical lymph node enlargement and can also have unusual presentation, such as axillary and inguinal lymph node enlargement, skin rash, arthralgia, splenomegaly, and aseptic meningitis [4]. KFD can even be complicated with fatal outcomes like disseminated intravascular coagulopathy (DIC), and pulmonary hemorrhage [14]. Patients with classic symptoms respond to NSAIDs or steroid, and those with severe symptoms usually respond to steroids. Immunosuppressants have been recommended for complicated case to prevent fatal outcome [15].
HC is widely used in diverse conditions and diseases. HC is used in immunomodulation, induction of apoptosis, lymphoid accumulation, and posttranslational viral glycosylation [16]. HC inhibits several enzymes and interferes with monocyte-macrophage, and suppresses the production of proinflammatory cytokines, such as interleukin (IL)-1, IL-6 at a posttranscriptional level. In a prospective study, serum IL-6 levels decreased 6-12 weeks after treatment of HC [17]. HC can induce apoptosis of effector T cells by inhibiting autophagy [18], and inhibit T cell antigen receptor signaling pathway through the calcium signal [19]. Impaired apoptosis of T cells can develop the autoimmunity. Also, HC make alkalization of acidic intracellular vesicles and inhibit the growth of the intracellular organism such as bacteria and virus [16]. A previous study has reported the use of HC with steroids when KFD is associated with SLE or other autoimmune diseases. However, in these cases, HC was used to decrease autoimmune-mediated inflammation rather than to treat KFD. A previous study conducted overseas reported the successful treatment of two episodes of KFD using only HC. Even without the use of steroids, HC led to a resolution of fever and systemic symptoms because it is associated with impairment of production of IFN-γ [20].
This result indicates the possibility of using HC for the treatment of KFD. However, there have been no reports about the use of HC for KFD treatment in Korea. Even studies from foreign countries are limited to case reports. In addition to the methods described above, there are case reports on the use of immunoglobulin, cyclosporine, minocycline, and ciprofloxacin for the treatment of patients with complicated KFD [614].
In our case report, the patient was suffering from a fourth rerecurrence of KFD. Even though the patient was treated with NSAIDs and prednisolone, cervical lymph node enlargement and pain persisted. Considering that KFD was not associated with SLE, HC at 3 mg/kg/day was used for symptom control. There are several case reports of complicated KFD does not respond to NSAIDs and steroids [814]. Furthermore, the overall prevalence rate of KFD is increasing in Korea [3]. This leads to a consideration about the use of HC for the treatment of KFD patients who have persistent symptoms, treated by NSAIDs and steroids. Therefore, we reported a case of a KFD patient who was unresponsive to NSAIDs and steroids, and experienced symptom resolution after treatment with HC.
Figures and Tables
 | Figure 1Multiple enlarged or borderline sized LNs in both level II, III, IV, Va, and Vb.Perinodal infiltration is noted around the enlarged lymph nodes(arrows). |
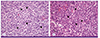 | Figure 3The excisional biopsies of cervical lymph nodes, show (A) aggregates of histiocytes (astra), nonphagocytic and phagocytic, with crescentic nuclei (H&E stains, ×100) and (B) similar findings consisting of many histiocytes with crescent-shaped nuclei (arrowheads) in necrotic background (H&E stains, ×400). |
References
1. Kikuchi M. Lymphadenitis showing focal reticulum cells hyperplasia with nuclear debris and phagocytosis: A clinicopathological study. Acta Hematol Jpn. 1972; 35:379–380.
2. Fujimoto Y, Kojima Y, Yamaguchi K. Cervical subacute necrotizing lymphadenitis. Naika. 1972; 30:920–927.
3. Song JY, Lee J, Park DW, Sohn JW, Suh SI, Kim IS, Kim WJ, Kim MJ, Cheong HJ. Clinical outcome and predictive factors of recurrence among patients with Kikuchi's disease. Int J Infect Dis. 2009; 13:322–326.

4. Bosch X, Guilabert A, Miquel R, Campo E. Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J Clin Pathol. 2004; 122:141–152.
5. Yoo IH, Na H, Bae EY, Han SB, Lee SY, Jeong DC, Kang JH. Recurrent lymphadenopathy in children with Kikuchi-Fujimoto disease. Eur J Pediatr. 2014; 173:1193–1199.

6. Kucukardali Y, Solmazgul E, Kunter E, Oncul O, Yildirim S, Kaplan M. Kikuchi-Fujimoto Disease: analysis of 244 cases. Clin Rheumatol. 2007; 26:50–54.

8. Rezai K, Kuchipudi S, Chundi V, Ariga R, Loew J, Sha BE. Kikuchi-Fujimoto disease: hydroxychloroquine as a treatment. Clin Infect Dis. 2004; 39:e124–e126.

9. Goldblatt F, Andrews J, Russell A, Isenberg D. Association of Kikuchi-Fujimoto's disease with SLE. Rheumatology (Oxford). 2008; 47:553–554.

10. Nayak HK, Mohanty PK, Mallick S, Bagchi A. Diagnostic dilemma: Kikuchi's disease or tuberculosis? BMJ Case Rep. 2013; 2013:pii:bcr2012008026.

11. Ram R, Swarnalatha G, Adiraju KP, Srinivasan VR, Dakshinamurty KV. Kikuchi–Fujimoto disease associated with systemic lupus erythematosus. NDT Plus. 2011; 4:238–240.

12. Ohshima K, Haraoka S, Takahata Y, Takada H, Tsutiya K, Suzuk K, Suzumiya J, Kikuchi M. Interferon-gamma, interleukin-18, monokine induced by interferon-gamma and interferon-gamma-inducible protein-10 in histiocytic necrotizing lymphadenitis. Leuk Lymphoma. 2002; 43:1115–1120.

13. Facchetti F, Vermi W, Mason D, Colonna M. The plasmacytoid monocyte/interferon producing cells. Virchows Arch. 2003; 443:703–717.

14. Uslu E, Gurbuz S, Erden A, Aykas F, Karagoz H, Karahan S, Karaman H, Cetinkaya A, Avci D. Disseminated intravascular coagulopathy caused by Kikuchi-Fujimoto disease resulting in death: first case report in Turkey. Int Med Case Rep J. 2014; 7:19–22.
15. Lin DY, Villegas MS, Tan PL, Wang S, Shek LP. Severe Kikuchi's disease responsive to immune modulation. Singapore Med J. 2010; 51:e18–e21.
16. Olsen NJ, Schleich MA, Karp DR. Multifaceted effects of hydroxychloroquine in human disease. Semin Arthritis Rheum. 2013; 43:264–272.

17. Barrera P, Boerbooms AM, van de Putte LB, van der Meer JW. Effects of antirheumatic agents on cytokines. Semin Arthritis Rheum. 1996; 25:234–253.

18. van Loosdregt J, Spreafico R, Rossetti M, Prakken BJ, Lotz M, Albani S. Hydroxychloroquine preferentially induces apoptosis of CD45RO+ effector T cells by inhibiting autophagy: a possible mechanism for therapeutic modulation of T cells. J Allergy Clin Immunol. 2013; 131:1443–1446.e1.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download