Abstract
Posaconazole is a new oral triazole with broad-spectrum antifungal activity. Posaconazole has also shown a significant advantage of preventing invasive fungal infection compared to fluconazole or itraconazole in patients with prolonged neutropenia. Indeed, posaconazole has been commonly used for antifungal prophylaxis in patients undergoing remission induction chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. We experienced a case of fatal mucormycosis despite posaconazole prophylaxis. To our knowledge, this is the first reported case of fatal breakthrough mucormycosis in a patient receiving posaconazole prophylaxis during remission induction chemotherapy in Korea. This case demonstrated that breakthrough fungal infection can occurs in patients receiving posaconazole prophylaxis because of its limited activity against some mucorales.
Administration of prophylactic antifungal agents has reduced the incidence of invasive fungal infection and improved the survival rate and prognosis in patients with prolonged neutropenia after chemotherapy [1]. Indeed, prophylactic antifungal agents are recommended to prevent invasive fungal infections in patients who are undergoing intensive chemotherapy for acute leukemia [2, 3]. Among antifungal agents, posaconazole has shown a significant advantage of preventing invasive fungal infection and improving overall survival compared to fluconazole or itraconazole in patients undergoing remission induction chemotherapy for acute myelogenous leukemia (AML) or myelodysplastic syndrome (MDS) despite its adverse effects [4]. Posaconazole is a new oral triazole with broad-spectrum antifungal activity against Candida spp., Aspergillus, and some of the members of the order Mucorales [5].
In United States of America, oral posaconazole has been approved for antifungal prophylaxis in patients ≥13 years who are at high risk of developing fungal infections because of immunosuppression, such as those with hematological malignancies who have sustained neutropenia as a result of chemotherapy [6]. In Korea also, posaconazole was approved as an antifungal prophylactic agent by the Korea Food and Drug Administration, and drug reimbursement coverage has been available since January 2013 for remission induction chemotherapy for AML or MDS. Although posaconazole has reduced the incidence of invasive fungal infections and mortality, a few cases of breakthrough invasive fungal infection among patients during posaconazole prophylaxis have been described in the literature [4, 7, 8, 9]. This article reports the first case of fatal fungal pneumonia due to mucormycosis despite posaconazole prophylaxis in Korea. Here, we describe the significance and limitations of posaconazole prophylaxis in patients with severe prolonged neutropenia. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital at the Catholic University of Korea with a waiver of informed consent (Subject number: KC13ZISE0629, Review date: Semptember 30, 2013).
A 67-year-old man who was admitted for fever was diagnosed with secondary AML. He had been diagnosed with a myeloproliferative disorder 1 year ago and was taking hydroxyurea. Piperacillin/tazobactam was started and the fever subsided. There was no evidence of bacterial or fungal infection. He underwent remission induction chemotherapy with idarubicin (30 mg/m2) for 3 days and cytosine arabinoside (100 mg/m2) for 5 days. Posaconazole (200 mg orally 3 times a day during meal) prophylaxis was started just before the first day of chemotherapy. He was taking posaconazole with food.
On day 12 after chemotherapy, neutropenic fever developed (axillary temperature of up to 38.4℃). Laboratory data showed a white blood cell count of 120/mm3 (absolute neutrophil count [ANC] 0/mm3), hemoglobin level of 7.0 g/dL, platelet count of 12,000/mm3, and C-reactive protein level of 14.5 mg/dL. Liver function enzymes were within their normal ranges (aspartate aminotransferase level of 7 U/L, alanine aminotransferase level of 13 U/L, alkaline phosphatase level of 46 U/L, gamma glutamyl transferase level of 49 U/L, and total bilirubin level of 0.84 mg/dL). Lobar pneumonia was found in the right upper lobe and superior segment of the right lower lobe on chest X-ray and low-dose computed tomography (CT) of the chest (Fig. 1A and B). The antifungal agent was changed from posaconazole to amphotericin B deoxycholate (1 mg/kg/day), and meropenem (1,000 mg three times a day) was added.
Because of aggravation of pneumonia on chest X-rays, the antifungal agent was changed to liposomal amphotericin B (5 mg/kg/day) on day 18, and teicoplanin (400 mg/day after being given 400 mg every 12 hours for 3 doses) was added on day 20. Also, bronchoscopy with bronchoalveolar lavage and biopsy were performed on day 21. In the bronchoscopic findings, abnormal mucosal lesions with easy touch bleeding were found in the right upper lobe and the right bronchus intermedius. There was no fungal, bacterial, or viral growth in the bronchial fluid and repetitive blood cultures. Repeated galactomannan antigen tests performed twice a week were consistently negative during the hospitalization period. Aggravation of pneumonia with pulmonary arterial invasion was found on follow-up chest X-ray and CT scan of the chest on day 26 (Fig. 1C and D, arrow). Despite treatment with antibacterial agents (meropenem and teicoplanin) and liposomal amphotericin B, he expired on day 27 due to massive pulmonary hemorrhage secondary to progressive pneumonia and thrombocytopenia.
Biopsy showed broad width, non-septate into broad, non-septated, non-parallel sided, non-dichotomous branching and ribbon like hyphae consistent with mucormycosis (Fig. 2).
A schematic diagram of the clinical courses of the patient is presented in Figure 3.
A few cases of breakthrough invasive fungal infection have been reported in the literature among allogeneic stem cell transplant patients during posaconazole prophylaxis [7, 8, 9]. But our case showed that breakthrough mucormycosis also occurs in patients receiving posaconazole prophylaxis during remission induction chemotherapy. And as compared with other literature [7, 8, 9], limitation of our case was that the mucorales species were not cultured from biopsy samples or bronchoalveolar lavage.
It seems that breakthrough mucormycosis could be occured in our patient despite of posaconazole prophylaxis due to the following reasons. First, posaconazole has limited activity against some of the mucorales species. Second, incidence of invasive fungal infection could be increased in lower plasma posaconazole concentration. Finally, serologic marker is not available for the early detection of mucormycosis.
Posaconazole is one of the oral antifungal agents with an extended spectrum of activity. Although posaconazole has clinical efficacy against the order mucorales, all of the Mucorales species are not sensitive to posaconazole [10]. In the literature, i.e., in the in vitro antifungal susceptibility data for 217 clinical isolates of the mucorales, 64-100% of the isolates were susceptible to posaconazole using a discretionary breakpoint of ≤0.5 ug/mL. Mucor circinelloides group had higher minimal inhibition concentration values and was the most resistant to posaconazole. Therefore, posaconazole is not recommended as primary treatment of mucormycosis. It could be a second line treatment option for patients with mucormycosis who are intolerant of or refractory to amphotericin B or who need extended continuation therapy [11]. In our case, it is possible that the patient was infected by the mucorales species that are resistant to posaconazole. But, this possibility was not confirmed on culture in our patient.
It is questionable whether monitoring of the plasma posaconazole concentration is needed for effective prophylaxis. Also, the optimal target drug levels for antifungal prophylaxis are not clearly defined. Although there are only a few studies, these reports suggested that there is a positive association between plasma posaconazole concentration and therapeutic antifungal efficiency. One multicenter study on salvage therapy with posaconazole for invasive aspergillosis reported that the elevated plasma posaconazole concentrations were associated with better response rates [12]. The pharmacokinetic data on posaconazole prophylaxis in allogeneic hematopoietic stem cell transplant recipients demonstrated that lower plasma posaconazole levels were detected in 5 patients who developed invasive fungal infection (median level 611 ng/mL) compared to patients without fungal infection (median level 922 ng/mL) [13]. Plasma posaconazole concentration tended to be low particularly in patients with gastrointestinal problems such as diarrhea and mucositis [14]. Therefore, monitoring of the plasma posaconazole concentration is necessary in neutropenic patients in whom gastrointestinal disorders occur frequently. Unfortunately, plasma posaconazole concentration was not measured in this study. However, the patient was taking posaconazole with meals, as was confirmed by the medical staff. Also, he did not have any symptoms of mucositis, vomiting and diarrhea that could possibly reduce drug absorption.
There is no serologic marker for the early detection of mucormycosis, and the antigen detection marker (galactomannan or beta-glucan) tests do not detect mucormycosis [11]. Although attempts have been made for molecular detection of the mucormycosis species [15, 16], these tests are not currently available. Therefore, it is essential to acquire biologic specimens from the infected site for establishing the diagnosis. Early confirmation of the diagnosis of mucormycosis could be achieved through demonstration of hyphae by direct microscopy and tissue culture from the specimens. In our case, repeated galactomannan antigen tests were consistently negative. Otherwise, we had to consider the possibility of a false-negative result of galactomannan antigen tests. This is because the sensitivity of galactomannan antigen test is decreased by antifungal prophylaxis with a mold-active agent [17].
Optimal approach for the treatment of mucormycosis is early treatment with an active fungal agent, controlling the underlying condition and surgical treatment of the tissue involved [11, 18]. First, early treatment with liposomal amphotericin B as the most active fungal agent is important in patients with mucormycosis resistant to posaconazole. In a retrospective review study of 70 patients with hematologic malignancy and mucormycosis, administration of amphotericin B deoxycholate (n = 12) or liposomal amphotericin B (n = 58) beyond 6 days after diagnosis resulted in poor mortality outcome [19]. In our patient, the antifungal agent was changed to amphotericin B deoxycholate at the time of diagnosis of lobar pneumonia. We then again changed the antifungal agent to liposomal amphotericin B after 7 days. Second, controlling the underlying condition is important. Our patient was in a state of persistent neutropenia after remission induction chemotherapy. Persistent prolonged profound neutropenia as the underlying condition in our patient could not be controlled despite treatment with granulocyte-colony stimulating factor. Third, extensive debridement of infected tissue is also important. In a retrospective review study of 255 patients with pulmonary mucormycosis, the mortality in patients treated with both surgically and medically was 11%, significantly lower than the 68% mortality in those treated medically alone [20]. However, in our patient, the surgical treatment of the lung lesion was impossible due to severe thrombocytopenia. Despite our efforts, he expired due to severe mucormycosis and pulmonary hemorrhage.
In conclusion, posaconazole can be used for antifungal prophylaxis during remission induction chemotherapy for AML or MDS with an excellent prophylactic effect. But, breakthrough mucormycosis can occur because posaconazole has limited activity against some of the mucorales species. Acquiring biologic specimens from the infected sites is essential for establishing the diagnosis of mucormycosis, and further research is needed for identifying a serologic marker for early detection of mucormycosis. Also, measuring the plasma posaconazole concentration may be necessary in patients in whom posaconazole is administered. Finally, it should be kept in mind that breakthrough fungal infection can occur in patients receiving posaconazole prophylaxis during chemotherapy.
Figures and Tables
Figure 1
Chest X-ray (A) and CT scan of the chest (B) showed lobar pneumonia in the upper lobe and the superior segment of the right lower lobe on day 12 after chemotherapy. Follow-up chest X-ray (C) and CT scan of the chest (D) showed aggravation of pneumonia with pulmonary arterial invasion (red arrow) on day 26.
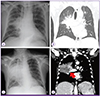
Figure 2
Microscopic analysis of the biopsy specimen showed broad width, non-septate, non-parallel sided, non-dichotomous branching and ribbon like hyphae consistent with mucormycosis (A: hematoxylin & eosin stain, ×400, B: periodic acid Schiff stain, ×400, C: Gomori methenamine silver stain, ×400).
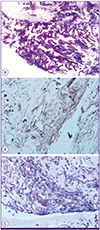
Figure 3
Schematic presentation of the patient's clinical course.
TEC, teicoplanin; TZP, piperacillin/tazobactam; MEM, meropenem; PSC, posaconazole; AMB, amphotericin B deoxycholate; LAB, liposomal amphotericin B; IVIG, intravenous immunoglobulin; G-CSF, granulocyte-colony stimulating factor; ANC, absolute neutrophil count.

References
1. Bow EJ, Laverdiere M, Lussier N, Rotstein C, Cheang MS, Ioannou S. Antifungal prophylaxis for severely neutropenic chemotherapy recipients: a meta analysis of randomized-controlled clinical trials. Cancer. 2002; 94:3230–3246.

2. Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011; 52:e56–e93.

3. Lee DG, Kim SH, Kim SY, Kim CJ, Min CK, Park WB, Park YJ, Song YG, Jang JS, Jang JH, Jin JY, Choi JH. Evidence-based guidelines for empirical therapy of neutropenic fever in Korea. Infect Chemother. 2011; 43:285–321.

4. Cornely OA, Maertens J, Winston DJ, Perfect J, Ullmann AJ, Walsh TJ, Helfgott D, Holowiecki J, Stockelberg D, Goh YT, Petrini M, Hardalo C, Suresh R, Angulo-Gonzalez D. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007; 356:348–359.

5. Rachwalski EJ, Wieczorkiewicz JT, Scheetz MH. Posaconazole: an oral triazole with an extended spectrum of activity. Ann Pharmacother. 2008; 42:1429–1438.

6. Frampton JE, Scott LJ. Posaconazole: a review of its use in the prophylaxis of invasive fungal infections. Drugs. 2008; 68:993–1016.
7. Mousset S, Bug G, Heinz WJ, Tintelnot K, Rickerts V. Breakthrough zygomycosis on posaconazole prophylaxis after allogeneic stem cell transplantation. Transpl Infect Dis. 2010; 12:261–264.

8. Schlemmer F, Lagrange-Xélot M, Lacroix C, de La Tour R, Socié G, Molina JM. Breakthrough Rhizopus infection on posaconazole prophylaxis following allogeneic stem cell transplantation. Bone Marrow Transplant. 2008; 42:551–552.

9. Lekakis LJ, Lawson A, Prante J, Ribes J, Davis GJ, Monohan G, Baraboutis IG, Skoutelis AT, Howard DS. Fatal rhizopus pneumonia in allogeneic stem cell transplant patients despite posaconazole prophylaxis: two cases and review of the literature. Biol Blood Marrow Transplant. 2009; 15:991–995.

10. Almyroudis NG, Sutton DA, Fothergill AW, Rinaldi MG, Kusne S. In vitro susceptibilities of 217 clinical isolates of zygomycetes to conventional and new antifungal agents. Antimicrob Agents Chemother. 2007; 51:2587–2590.

11. Skiada A, Lanternier F, Groll AH, Pagano L, Zimmerli S, Herbrecht R, Lortholary O, Petrikkos GL. European Conference on Infections in Leukemia. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 2013; 98:492–504.

12. Walsh TJ, Raad I, Patterson TF, Chandrasekar P, Donowitz GR, Graybill R, Greene RE, Hachem R, Hadley S, Herbrecht R, Langston A, Louie A, Ribaud P, Segal BH, Stevens DA, van Burik JA, White CS, Corcoran G, Gogate J, Krishna G, Pedicone L, Hardalo C, Perfect JR. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis. 2007; 44:2–12.

13. Krishna G, Martinho M, Chandrasekar P, Ullmann AJ, Patino H. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy. 2007; 27:1627–1636.

14. Lebeaux D, Lanternier F, Elie C, Suarez F, Buzyn A, Viard JP, Bougnoux ME, Lecuit M, Jullien V, Lortholary O. Therapeutic drug monitoring of posaconazole: a monocentric study with 54 adults. Antimicrob Agents Chemother. 2009; 53:5224–5229.

15. Dannaoui E. Molecular tools for identification of Zygomycetes and the diagnosis of zygomycosis. Clin Microbiol Infect. 2009; 15:Suppl 5. 66–70.

16. Dannaoui E, Schwarz P, Slany M, Loeffler J, Jorde AT, Cuenca-Estrella M, Hauser PM, Shrief R, Huerre M, Freiberger T, Gaustad P, Rodriguez-Tudela JL, Bille J, Denning DW, Bretagne S, Lortholary O. Molecular detection and identification of zygomycetes species from paraffin-embedded tissues in a murine model of disseminated zygomycosis: a collaborative European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Fungal Infection Study Group (EFISG) evaluation. J Clin Microbiol. 2010; 48:2043–2046.

17. Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clin Infect Dis. 2005; 40:1762–1769.

18. Hong HL, Lee YM, Kim T, Lee JY, Chung YS, Kim MN, Kim SH, Choi SH, Kim YS, Woo JH, Lee SO. Risk factors for mortality in patients with invasive mucormycosis. Infect Chemother. 2013; 45:292–298.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download